The development of anti-Aspergillus fumigatus CAR T-cell therapy represents a promising frontier in the fight against invasive aspergillosis (IA), a severe fungal infection particularly dangerous for immunocompromised patients. Current antifungal therapies often have limited efficacy, highlighting the urgent need for novel immune-enhancing strategies. Creative Biolabs provides a specialized CellRapeutics™ anti-Aspergillus fumigatus CAR cell therapy development service to develop effective therapeutics to assist in health safeguard.
Invasive fungal infections (IFIs), particularly those caused by Aspergillus fumigatus, pose a significant threat to immunocompromised patients, leading to high mortality rates despite current antifungal treatments. The emergence of drug resistance and the systemic toxicities associated with existing therapies underscore an urgent unmet medical need for novel, targeted interventions. Anti-Aspergillus fumigatus CAR cell therapy represents a paradigm shift, offering a precise immunotherapeutic approach to combat these life-threatening infections by specifically targeting fungal pathogens, thereby enhancing patient outcomes and overcoming the limitations of conventional treatments.
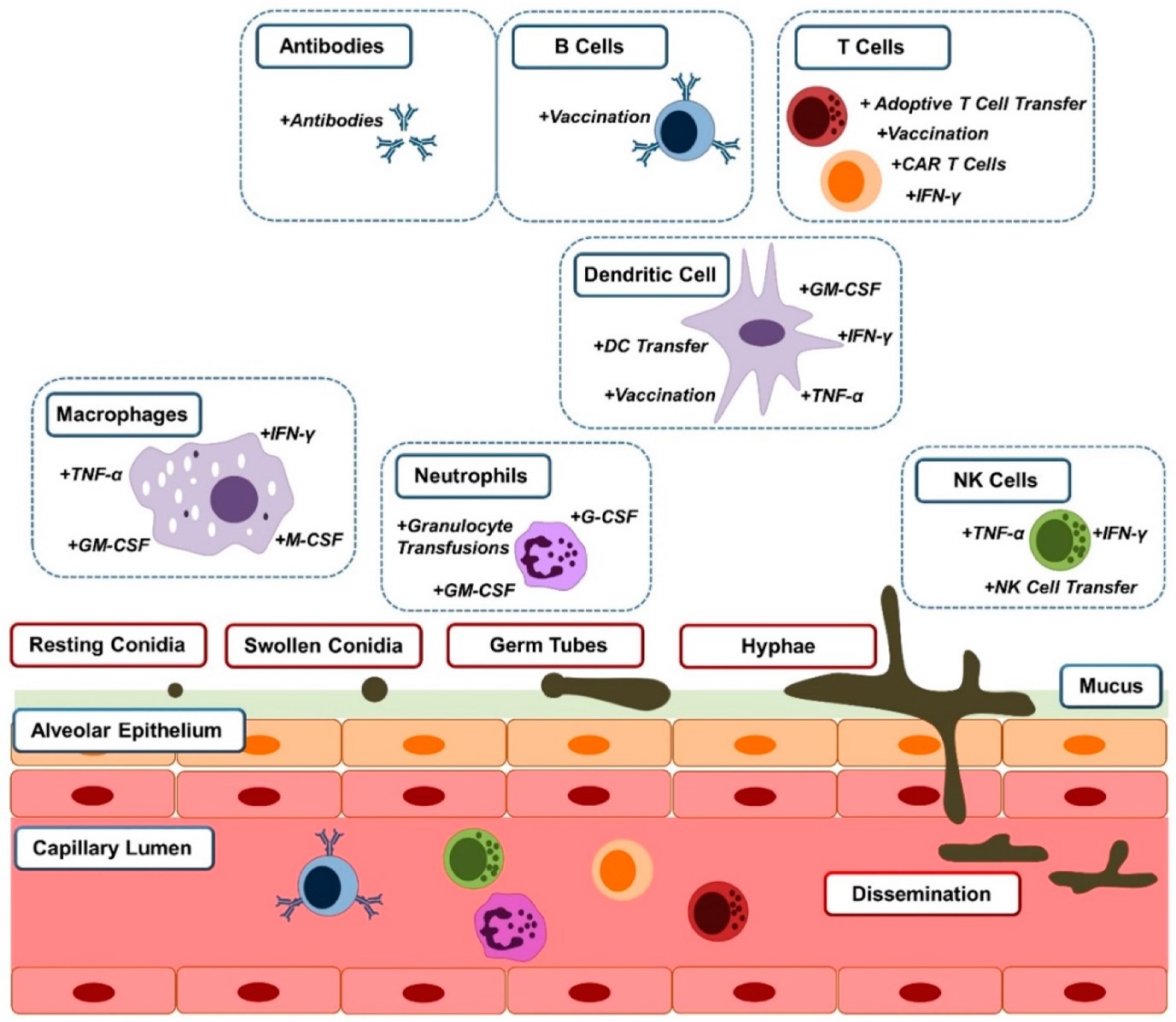 Fig.1 The interaction of Aspergillus fumigatus with innate and adaptive immune cells.1
Fig.1 The interaction of Aspergillus fumigatus with innate and adaptive immune cells.1
Creative Biolabs offers a comprehensive solution for developing cutting-edge CAR cell therapies specifically designed to target Aspergillus fumigatus. Our service provides you with custom-engineered immune cells, robust preclinical validation data, and expert guidance to advance your therapeutic candidates. We deliver highly specific and potent CAR constructs, engineered immune cells ready for preclinical evaluation, and comprehensive data packages to support your research and development milestones.
We begin by collaborating with you to identify and validate highly specific and accessible Aspergillus fumigatus antigens. This involves bioinformatic analysis, literature review, and in vitro screening to ensure optimal target selection for CAR recognition.
Our experts design and optimize the chimeric antigen receptor (CAR) construct. This includes selecting the optimal single-chain variable fragment (scFv) for antigen binding, appropriate hinge and transmembrane domains, and intracellular signaling domains (e.g., CD3ζ, 4-1BB, CD28) to ensure robust activation and persistence of the engineered cells.
We utilize advanced viral vector technologies (e.g., lentiviral or retroviral systems) to efficiently deliver the CAR gene into immune cells. Our state-of-the-art facilities ensure high-titer, high-quality viral vector production for efficient transduction.
Patient-derived or donor immune cells (e.g., T cells, NK cells) are isolated, activated, and transduced with the optimized CAR construct. Following transduction, these CAR-expressing cells are expanded ex vivo to achieve the necessary cell numbers and purity for preclinical evaluation.
The modified CAR cells undergo rigorous preclinical testing to determine their potency, specificity, and safety. This includes in vitro assays (e.g., cytokine release, cytotoxicity, proliferation in response to A. fumigatus antigens) and in vivo studies using relevant fungal infection models to evaluate therapeutic potential.
Throughout the process, stringent quality control measures are implemented to ensure the purity, viability, and functionality of the CAR cell product. We also provide comprehensive documentation and data packages to support your regulatory submissions.
Effective CAR cell therapy relies on the precise identification of target antigens. For Aspergillus fumigatus, potential antigens for CAR recognition include:
Case Study One---
A leading academic research institution partnered with Creative Biolabs to develop a novel CAR construct targeting a unique Aspergillus fumigatus cell wall component. Through our Anti-Aspergillus fumigatus CAR cell therapy development service, the institution successfully identified multiple potential CAR targets and developed lead CAR constructs with demonstrated in vitro specificity and efficacy within a rapid 14-week timeframe, significantly accelerating their research.
Case Study Two---
A biopharmaceutical company sought to advance their anti-fungal CAR cell candidate into preclinical testing. After utilizing our service for CAR cell engineering and in vivo validation, the biopharmaceutical company successfully completed critical preclinical milestones, demonstrating significant reduction in fungal burden and improved survival in a murine model of invasive aspergillosis, paving the way for IND-enabling studies.
Q1: What types of immune cells can be engineered using your service?
A1: Our service is highly flexible and can engineer various immune cell types, primarily T cells and Natural Killer (NK) cells, to express the anti-Aspergillus fumigatus CAR. We can discuss which cell type is most suitable for your specific project goals during your consultation.
Q2: How long does the development process for an anti-Aspergillus fumigatus CAR cell therapy typically take?
A2: The estimated timeframe for our service generally ranges from 12 to 20 weeks. This duration can vary depending on the complexity of the CAR design, the extent of preclinical validation required, and the specific antigen targets involved. We provide a detailed project timeline after initial consultation.
Q3: What are the key safety considerations for anti-Aspergillus fumigatus CAR cell therapy, and how does Creative Biolabs address them?
A3: As with any cellular therapy, potential safety considerations include cytokine release syndrome (CRS) and potential off-target effects. To reduce these risks, Creative Biolabs conducts thorough in vitro and in vivo preclinical research, which includes specificity assays and toxicity studies. Our improved CAR designs seek to be highly selective to Aspergillus fumigatus antigens while reducing interactions with host cells.
Aside from this, Creative Biolabs offers a full array of ancillary services aimed at enhancing your research and speeding the development of breakthrough cellular immunotherapies and fungal immunology solutions:
Creative Biolabs stands at the forefront of CAR cell therapy development, offering unmatched expertise in fungal immunology and advanced cell engineering. Our commitment to innovation, coupled with our state-of-the-art platforms, ensures the highest quality and most effective solutions for your anti-fungal CAR cell therapy projects. If you want to know more about our CellRapeutics™ anti-Aspergillus fumigatus CAR cell therapy development service, please feel free to get in touch with us.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION

