To address persistent fungal infections, limited treatment options, and the challenge of drug resistance in immunocompromised patients, Creative Biolabs delivers a cost-effective CellRapeutics™ anti-Cryptococcus neoformans CAR Cell therapy development service that helps you develop highly effective and specific CAR cell therapies to combat this critical pathogen through advanced CAR engineering, robust cell manufacturing, and comprehensive preclinical validation.
Cryptococcus neoformans(C. neoformans) remains a significant global health threat, particularly for immunocompromised individuals, causing severe meningoencephalitis and pulmonary infections. Current antifungal treatments often face limitations such as drug resistance, toxicity, and inadequate efficacy in severe cases. Gene therapy, specifically Chimeric Antigen Receptor (CAR) T-cell therapy, has revolutionized cancer treatment and is now emerging as a promising strategy for infectious diseases. Research highlights the potential of CAR T cells to target fungal pathogens like C. neoformans by recognizing specific fungal antigens, offering a novel, highly targeted, and potent fungicidal approach to overcome the shortcomings of conventional therapies.
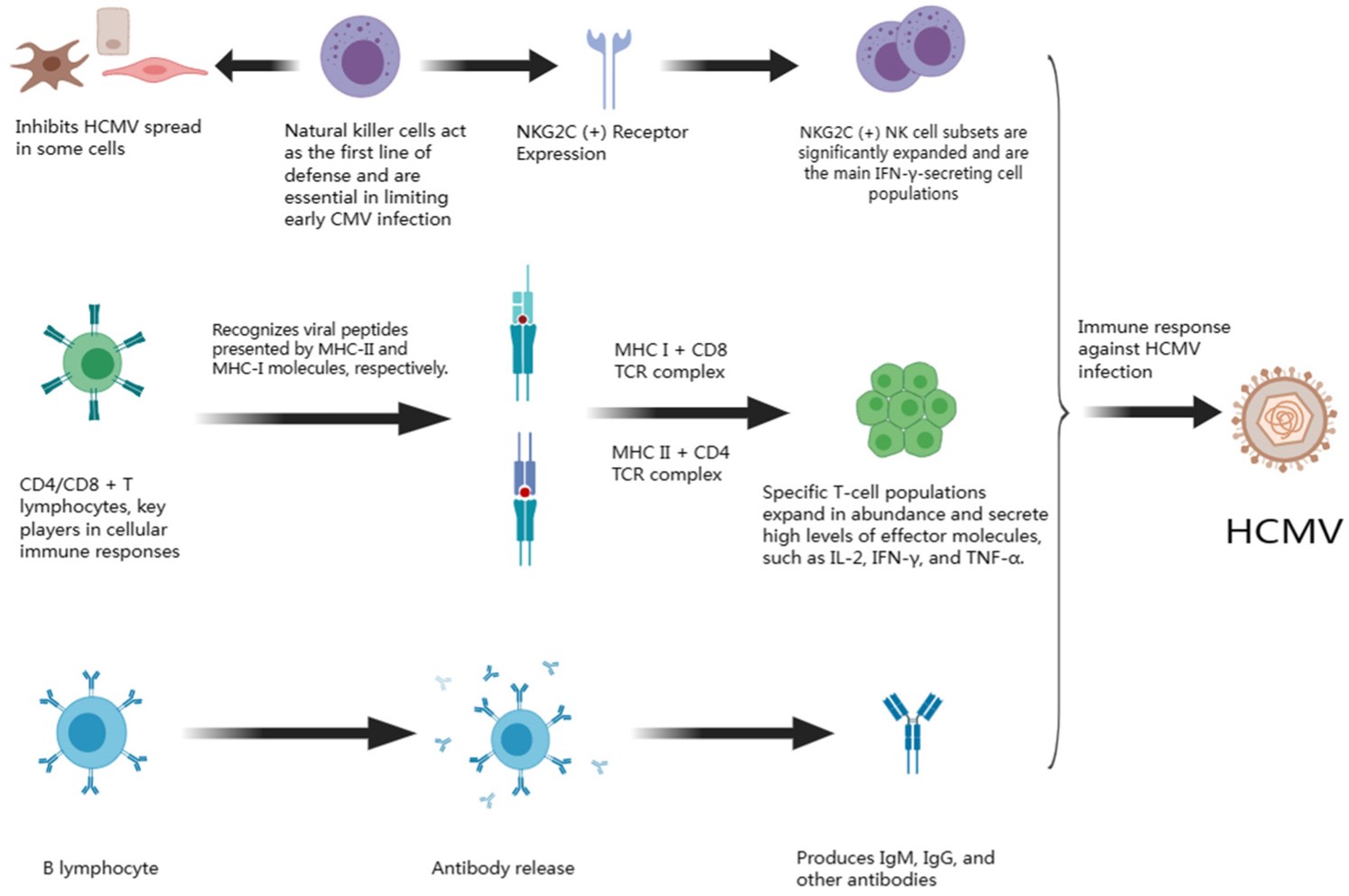 Fig.1 Cryptococcal virulence factors are key mechanisms that manipulate the host's immune response.1
Fig.1 Cryptococcal virulence factors are key mechanisms that manipulate the host's immune response.1
Creative Biolabs' CellRapeutics™ anti-C. neoformans CAR cell therapy development service delivers a comprehensive, end-to-end solution for the development of targeted cellular immunotherapies engineered to combat the challenging fungal pathogen, C. neoformans. This service encompasses expert custom CAR construct design, state-of-the-art high-quality CAR cell production, and robust in vitro and in vivo efficacy testing. Through these integrated capabilities, clients receive a meticulously developed, potent, and highly specific therapeutic candidate ready for further preclinical and clinical advancement.
Our experts assist in identifying optimal C. neoformans-specific antigens that are accessible and critical for fungal survival or virulence. Based on this, we design CAR constructs, selecting appropriate single-chain variable fragments (scFvs), hinge regions, transmembrane domains, and intracellular signaling domains (e.g., CD3ζ, 4-1BB, CD28) to ensure optimal specificity and robust T-cell activation.
The designed CAR construct is cloned into high-quality viral vectors, typically lentiviral or retroviral, known for their efficient gene transfer capabilities. We then proceed with the large-scale production of these recombinant viral vectors, ensuring high titers and purity suitable for cell transduction.
Immune cells (e.g., T cells, NK cells) are isolated, activated, and then transduced with the CAR-encoding viral vectors. Following successful transduction, the CAR-expressing cells are expanded ex vivo under optimized conditions to achieve the desired cell numbers while maintaining viability, phenotype, and functionality. Rigorous quality control checks are performed throughout the expansion phase.
Primary human T cells are isolated, activated, transduced with the CAR-expressing viral vectors, and expanded to therapeutic numbers under optimized conditions, ensuring high viability and transduction efficiency
The engineered CAR cells undergo comprehensive in vitro functional assays. This includes assessing their specificity against C. neoformans and non-target cells, evaluating their fungicidal activity (e.g., fungal killing assays, growth inhibition assays), cytokine secretion profiles upon antigen encounter, and proliferation capabilities.
For advanced projects, we offer in vivo preclinical efficacy assessment using relevant animal models of cryptococcosis. This involves administering the CAR cells to infected models and monitoring fungal burden reduction in various organs, survival rates, and potential off-target toxicities or adverse events.
The success of CAR cell therapy hinges on the identification of specific and accessible antigen targets on the surface of C. neoformans. Ideal targets are those that are consistently expressed by the pathogen, critical for its survival or virulence, and minimally expressed on host cells to avoid off-target toxicity. Key potential antigen targets for engineering anti-C. neoformans CAR cells include:
Q1: How long does the development process typically take, and what are the key milestones?
A1: The project timeline can vary based on complexity, but generally ranges from 12 to 24 weeks, encompassing stages from CAR construct design and cell manufacturing to comprehensive in vitro and in vivo validation. We provide regular updates and detailed reports at each milestone. Please reach out for a customized project proposal and timeline.
Q2: Can Creative Biolabs assist with preclinical validation of the developed CAR cell therapies?
A2: Absolutely. Our service includes robust in vitro functional assays and in vivo efficacy assessments in relevant animal models of cryptococcosis to thoroughly evaluate the therapeutic potential and safety profile of your CAR cell candidate. We welcome you to discuss your specific preclinical needs with our scientific team.
To further support your research and development goals, Creative Biolabs offers a range of complementary services that can synergize with your anti-C. neoformans CAR cell therapy project:
At Creative Biolabs, we are dedicated to advancing your anti-C. neoformansCAR cell therapy project with unparalleled expertise and cutting-edge facilities. Our dedicated scientific team brings deep knowledge in immunology, mycology, and gene therapy, ensuring a comprehensive and robust approach from concept to preclinical validation. We uphold stringent quality control throughout our pipeline, guaranteeing high-quality, functional CAR cell products. Please feel free to get in touch with us to discover how our service accelerates your meaningful projects.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION

