Precision engineering for specific EBV-associated targets, maximizing therapeutic impact.
Are you currently facing long drug development cycles, difficulty in targeting virally-associated malignancies, or challenges in developing effective and safe cell therapies? Creative Biolabs' CellRapeutics™ anti-EBV CAR cell therapy development service helps you accelerate drug discovery, obtain high-quality anti-EBV CAR-T cell therapies, and develop highly specific and potent cellular immunotherapies through advanced chimeric antigen receptor (CAR) engineering, high-throughput screening platforms, and innovative cell manufacturing techniques.
Epstein-Barr Virus (EBV) is linked to a spectrum of human malignancies, including lymphomas and nasopharyngeal carcinoma, posing significant challenges for conventional treatments. While CAR T-cell therapy has revolutionized hematological cancer treatment, novel approaches are urgently needed for EBV-associated B-cell malignancies, which often have poor outcomes with chemotherapy. Recent advancements show promise in engineering EBV-specific T cells with CARs, offering an "off-the-shelf" allogeneic platform with robust antitumor efficacy and reduced alloreactivity. This highlights the critical need for developing targeted therapies like anti-EBV CAR T cells to address these persistent unmet medical needs.
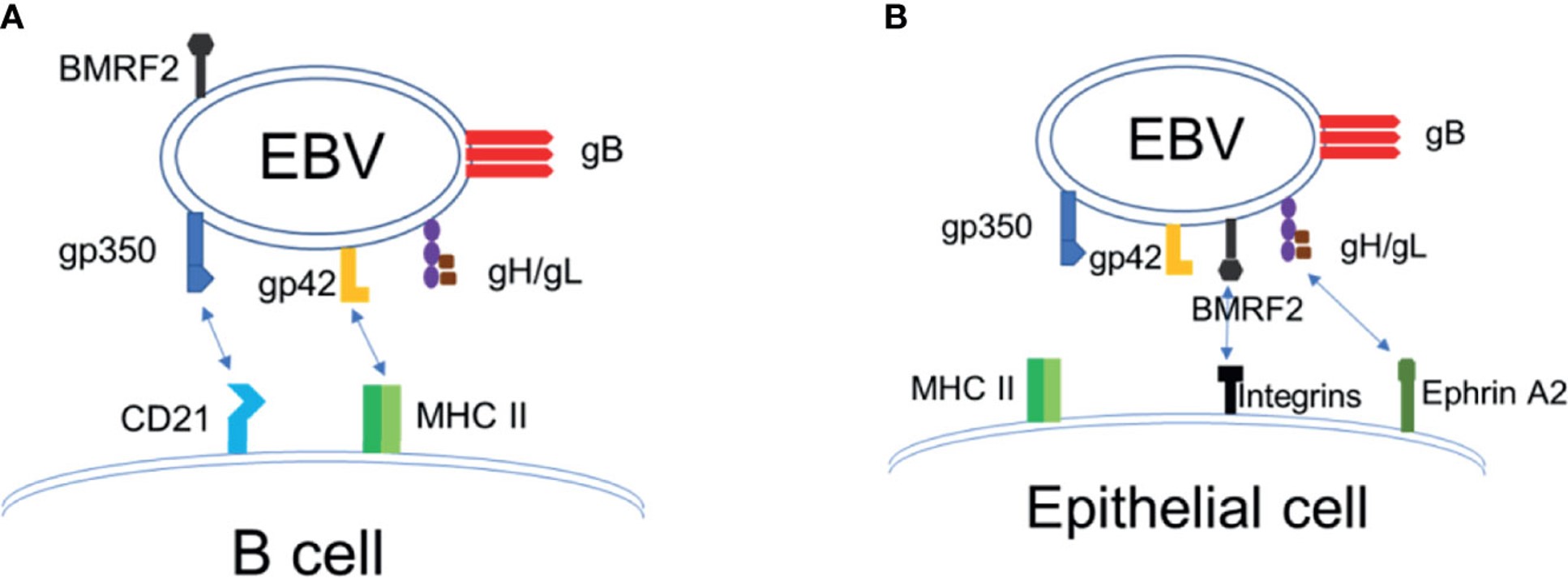 Fig.1 EBV enters and infects target cells.1,3
Fig.1 EBV enters and infects target cells.1,3
Our CellRapeutics™ anti-EBV CAR cell therapy development service provides comprehensive solutions tailored to your unique research and clinical needs, from concept to validated therapeutic candidates. We specialize in overcoming the complexities of CAR-T design, optimization, and manufacturing for EBV-associated indications. Our deliverables include expertly designed CAR constructs, robustly expanded and characterized CAR-T cell products, and in-depth functional and safety profiles. We help you accelerate your project by providing high-quality, reliable results that pave the way for successful preclinical and clinical development.
Based on your target antigen information, we design and synthesize highly efficient CAR constructs. This involves selecting optimal scFv domains, hinge and transmembrane regions, and co-stimulatory domains (e.g., CD28, 4-1BB, or novel combinations) to enhance T cell activation, proliferation, and persistence.
High-titer lentiviral or retroviral vectors encoding the optimized CAR are produced. We then efficiently transduce the client-provided or sourced T cells, ensuring stable integration and high expression of the CAR on the cell surface. Rigorous quality control checks are performed for vector integrity and transduction efficiency.
Transduced CAR-T cells are expanded under GMP-compliant conditions in our state-of-the-art facilities. Our optimized protocols ensure robust cell proliferation while maintaining desired phenotypes and functional characteristics, producing sufficient quantities for preclinical or clinical applications.
Expanded CAR-T cells undergo comprehensive in vitro testing. This includes antigen-specific cytotoxicity assays (e.g., luciferase-based killing, impedance-based assays), cytokine secretion profiling (e.g., IFN-γ, IL-2), proliferation assays, and assessment of exhaustion markers.
For projects requiring preclinical validation, we offer in vivo studies using appropriate animal models (e.g., xenograft models of EBV-associated malignancies) to evaluate CAR-T cell persistence, anti-tumor efficacy, and potential off-target toxicities, providing crucial data for IND-enabling studies.
Developing effective anti-EBV CAR T-cell therapies relies on identifying and targeting specific EBV antigens expressed on malignant cells. Here we list several potential targets:
Precision engineering for specific EBV-associated targets, maximizing therapeutic impact.
Streamlined workflows accelerate your CAR-T cell therapy from concept to preclinical readiness.
Utilize our expertise in CAR design to achieve superior specificity and potency.
High-quality, scalable production of CAR-T cells under stringent conditions.
In-depth functional and safety profiling ensures reliable and predictable outcomes.
Our proven methodologies minimize potential off-target effects and enhance overall safety.
Efficient processes and optimized resource utilization deliver exceptional value for your investment.
This study engineered CAR-T cells targeting the gp350 glycoprotein and evaluated their therapeutic potential against Epstein-Barr virus (EBV)-positive tumors. The gp350-CAR-T cells demonstrated potent and selective cytotoxicity against EBV-positive tumor cell lines in vitro and significantly inhibited tumor growth in an in vivo xenograft model of Burkitt lymphoma. These findings highlight gp350 as a promising and specific target for immunotherapy in EBV-associated malignancies.
Cytotoxicity Assessment
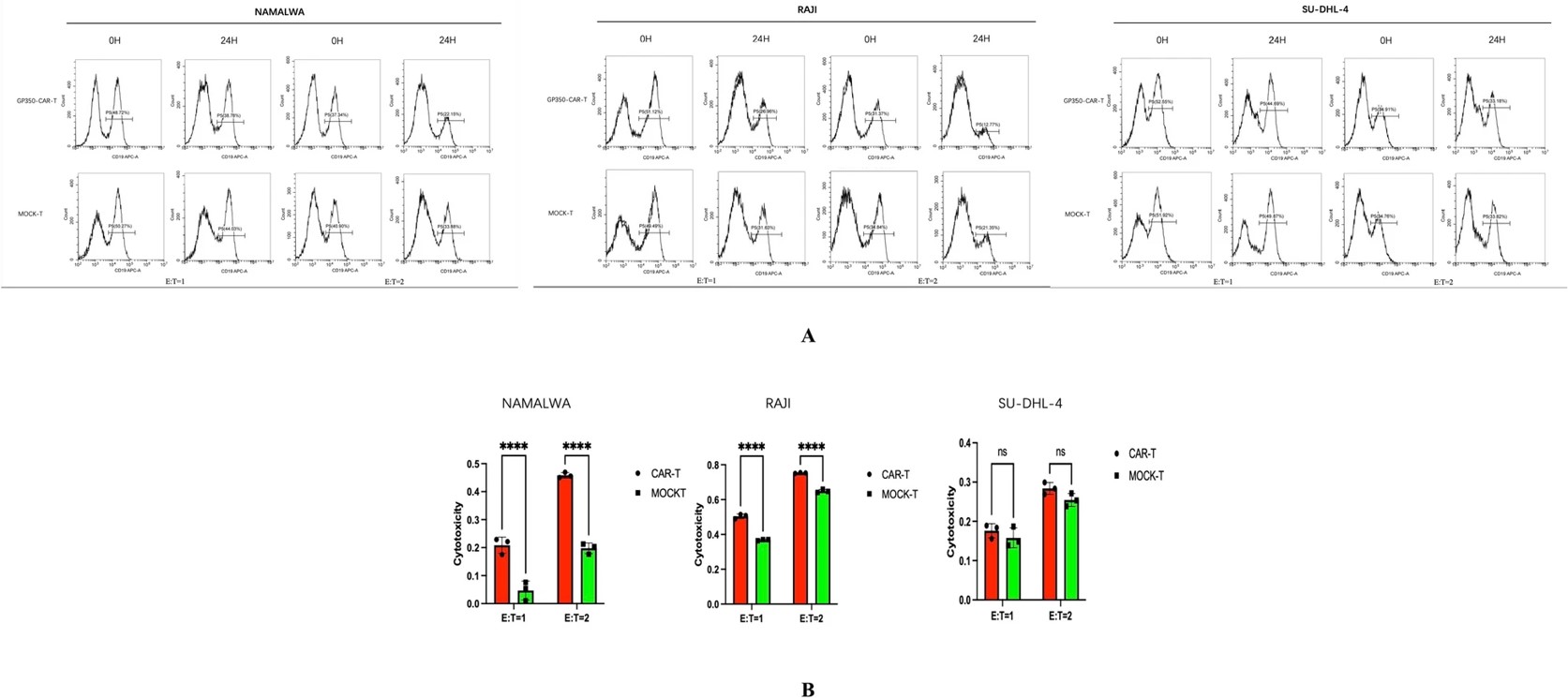 Fig.2 Anti-EBV CAR-T cell cytotoxicity assessment.2,3
Fig.2 Anti-EBV CAR-T cell cytotoxicity assessment.2,3
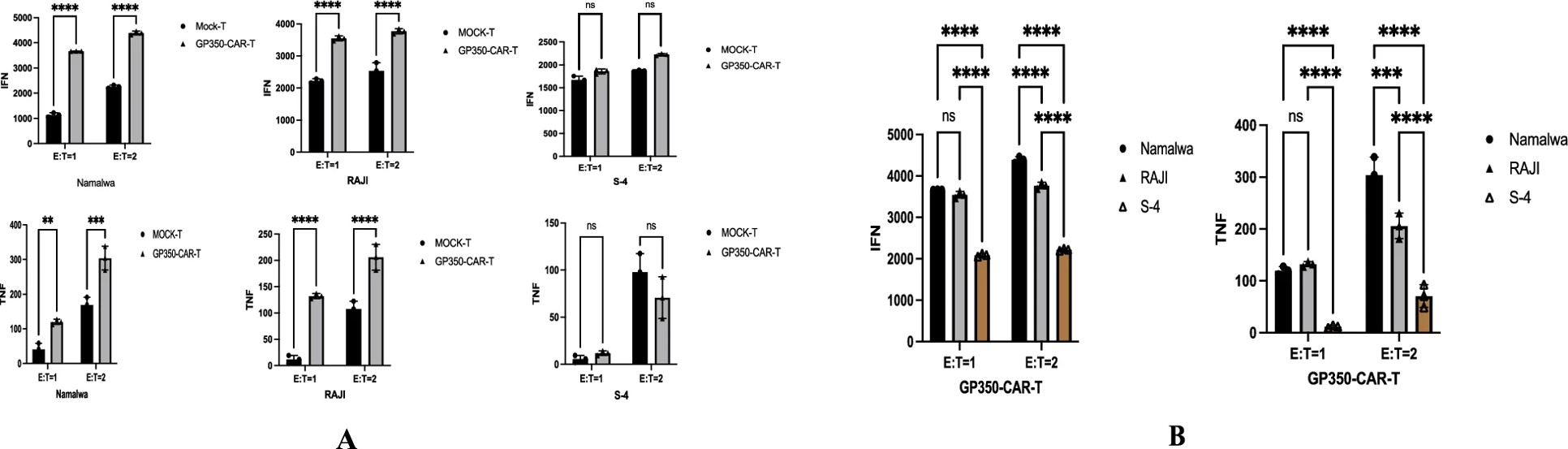 Fig.3 Anti-EBV CAR-T cell cytokine secretion assessment.2,3
Fig.3 Anti-EBV CAR-T cell cytokine secretion assessment.2,3
Q1: What types of EBV-associated malignancies can your Anti-EBV CAR Cell Therapy target?
A1: Our service can develop CAR-T cell therapies targeting various EBV-associated malignancies, including but not limited to EBV-positive lymphomas (e.g., Burkitt lymphoma, Hodgkin lymphoma, NK/T-cell lymphoma), post-transplant lymphoproliferative disorders (PTLD), and nasopharyngeal carcinoma. We work closely with clients to define the most appropriate antigen targets for their specific indications. Feel free to discuss your specific project with our experts for tailored advice.
Q2: Can you accommodate both autologous and allogeneic CAR-T cell development?
A2: Yes, our CellRapeutics™ platform is versatile and equipped to handle both autologous (patient-derived) and allogeneic (healthy donor-derived, "off-the-shelf") CAR-T cell development. We offer expertise in optimizing protocols for each approach, addressing the unique challenges associated with cell sourcing, expansion, and alloreactivity management for allogeneic platforms. Let us know your preferred strategy, and we'll guide you through the process.
To further support your immuno-oncology and cell therapy endeavors, Creative Biolabs offers a suite of complementary services:
At Creative Biolabs, our CellRapeutics™ anti-EBV CAR cell therapy development service is built upon a foundation of scientific excellence, cutting-edge technology, and an unwavering commitment to client success. We pride ourselves on our ability to navigate the intricacies of CAR-T engineering, offering bespoke solutions that address the unique challenges of each project. If you are looking for a reliable partner to develop high-quality anti-EBV CAR cell therapeutics, please feel free to reach out to us.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION

