Benefit from meticulously designed CAR constructs optimized for precise targeting of HCMV antigens.
Are you currently facing long development cycles for cell therapies, difficulty targeting persistent viral infections, challenges in achieving durable anti-viral responses in immunocompromised patients, or complex clinical trials for advanced therapies? Our CellRapeutics™ anti-HCMV CAR cell therapy development service helps you accelerate cell therapy development, obtain highly specific anti-HCMV CAR-T cells, and achieve robust and durable anti-viral responses through advanced CAR engineering, high-throughput screening platforms, and innovative cell manufacturing techniques.
Human Cytomegalovirus (HCMV) infection remains a significant cause of morbidity and mortality, particularly in immunocompromised patients following organ or hematopoietic stem cell transplantation. Despite existing antiviral therapies, limitations such as drug toxicity, resistance development, and the persistence of latent virus highlight a critical unmet medical need. Current gene therapy advancements, especially in chimeric antigen receptor (CAR) T-cell technology, offer a transformative approach. Leveraging CAR T-cells to specifically target HCMV antigens, such as pp65, holds immense promise to provide highly potent, specific, and durable antiviral immunity, significantly improving patient outcomes where conventional treatments fall short.
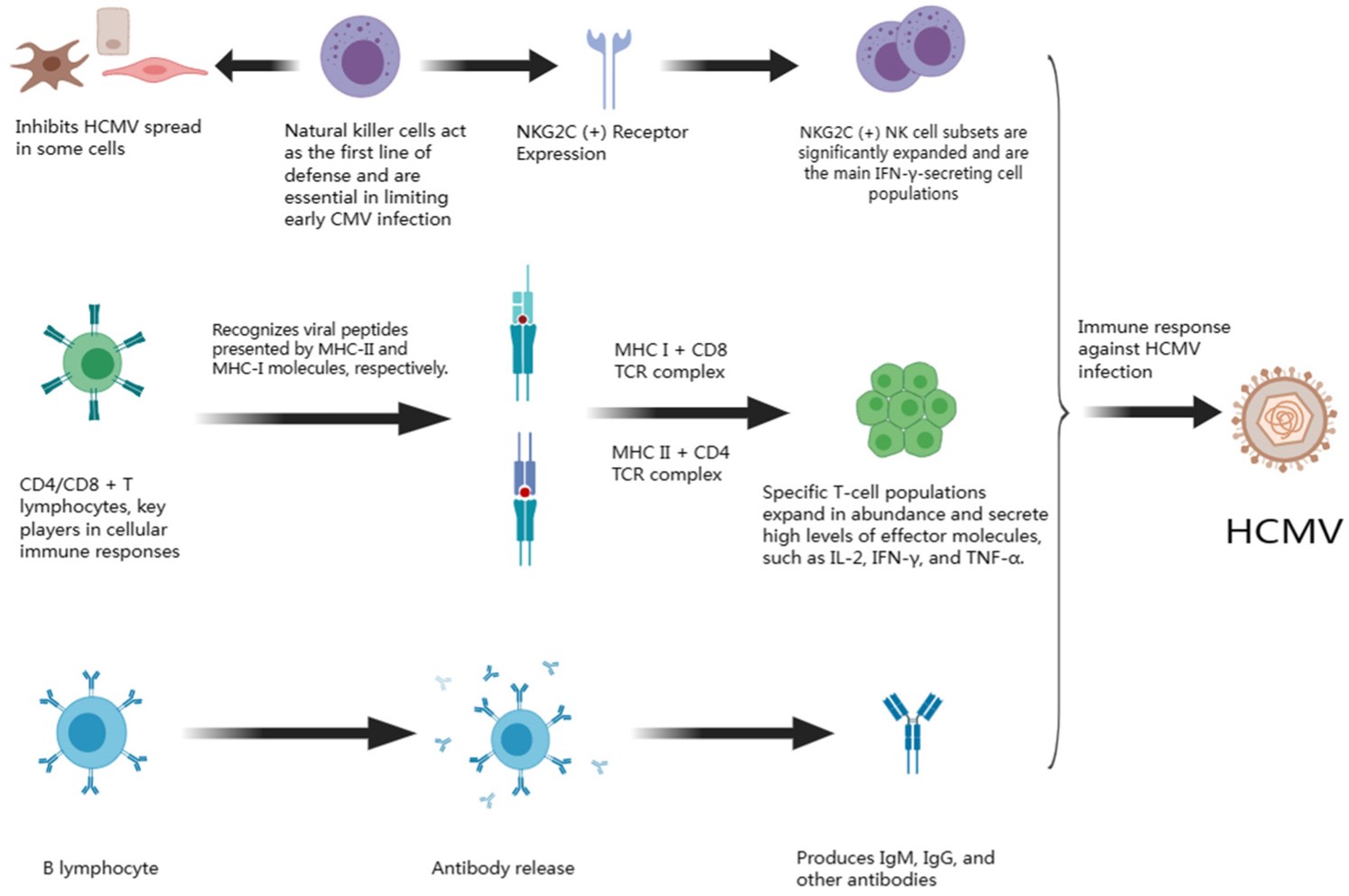 Fig.1 Immune response caused by HCMV infection.1
Fig.1 Immune response caused by HCMV infection.1
Creative Biolabs provides comprehensive solutions for the development of highly effective anti-HCMV CAR cell therapies, tailored to your specific project needs. We deliver robust preclinical data and cell products designed to accelerate your therapeutic pipeline.
We begin by performing in-depth analysis and validation of selected HCMV target antigens to ensure optimal specificity and accessibility for CAR binding.
Our experts design and construct custom CAR constructs, optimizing signaling domains (e.g., CD3ζ, 4-1BB, CD28) and scFv linkers for enhanced specificity, affinity, and T-cell activation. This involves gene synthesis and cloning into appropriate viral vectors.
High-titer, clinical-grade viral vectors (e.g., lentivirus, retrovirus) are produced and rigorously characterized for purity, potency, and safety.
Primary human T cells are isolated, activated, transduced with the CAR-expressing viral vectors, and expanded to therapeutic numbers under optimized conditions, ensuring high viability and transduction efficiency
Comprehensive in vitro assays are performed to assess CAR-T cell function, including antigen-specific cytotoxicity, cytokine secretion, proliferation, and persistence. Off-target toxicity is thoroughly evaluated.
For advanced projects, we conduct in vivo studies using relevant animal models to evaluate the therapeutic efficacy, persistence, and safety of the CAR-T cells in a living system.
Effective CAR T-cell therapy against HCMV relies on the precise identification and targeting of viral antigens expressed on infected cells. Key HCMV antigens that serve as promising targets for CAR development include:
Benefit from meticulously designed CAR constructs optimized for precise targeting of HCMV antigens.
Streamlined workflows and expert methodologies help significantly reduce your development schedule.
Robust preclinical data and optimized therapeutic candidates improve prospects in clinical trials.
Achieve sustained and effective anti-viral activity, especially crucial for immunocompromised patients.
Receive meticulously prepared documentation to support your regulatory submissions.
Q1: Can your service accommodate different T-cell sources or patient-specific requirements?
A1: Absolutely! Our service is highly flexible. We can work with various T-cell sources, including peripheral blood mononuclear cells (PBMCs) from healthy donors or specific patient populations, based on your project's needs. We can also tailor aspects of the development to fit unique patient-specific considerations. Let's discuss your particular requirements.
Q2: What is the typical turnaround time for an anti-HCMV CAR T-cell development project?
A2: The timeframe can vary depending on the scope and complexity of your project, typically ranging from 12 to 24 weeks for comprehensive preclinical development. We strive to deliver efficient and timely results without compromising quality. For a more precise estimate tailored to your project, please reach out for a detailed consultation.
To further support your research and development in cell and gene therapy, Creative Biolabs offers a series of complementary services:
Creative Biolabs is a global leader in cell and gene therapy development, offering an unparalleled commitment to innovation and client success. Our CellRapeutics™ anti-HCMV CAR cell therapy development service leverages decades of expertise and state-of-the-art platforms to deliver robust, high-quality solutions. We welcome global clients to partner with us to overcome complex challenges in CAR-T engineering and accelerate your journey from concept to therapeutic reality.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION

