Creative Biolabs provides an antigen-pulsed dendritic cell (DC) development service that leverages precision loading with peptides and proteins together with optimized DC maturation protocols to accelerate immunotherapy research, enable the generation of highly effective DC vaccines, and streamline development for diverse immunotherapy applications.
Dendritic cells (DCs) are the central activators of adaptive immunity, uniquely capable of initiating potent antigen-specific T-cell responses. Ex vivo pulsing, where DCs are loaded with specific antigens outside the body and then reintroduced, is a cornerstone strategy for therapeutic cancer vaccines. This approach has gained significant traction due to its ability to precisely direct immune responses.
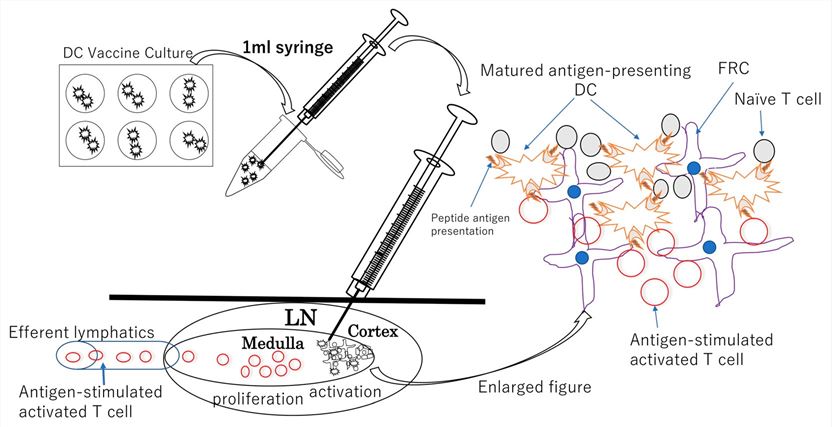 Fig.1 Schematic illustration of DC vaccine injection procedure.1
Fig.1 Schematic illustration of DC vaccine injection procedure.1
Creative Biolabs provides customized, high-quality antigen-pulsed DCs designed to meet your specific project requirements, from fundamental research to preclinical studies. Our expertise covers the entire workflow, including precise antigen selection—whether focusing on defined peptides, comprehensive full-length proteins, or personalized neoantigens derived from advanced sequencing.
You can expect optimized protocols for the generation, maturation, and antigen loading of DCs, ensuring maximum efficacy and consistent results. We employ rigorous quality control and thorough characterization processes, utilizing advanced techniques like flow cytometry and functional assays to guarantee the potency and functionality of every DC batch. Our scalable manufacturing capabilities are designed to facilitate a seamless transition from laboratory bench to clinical translation, supporting your project at every stage.
By partnering with Creative Biolabs, you gain the ability to:
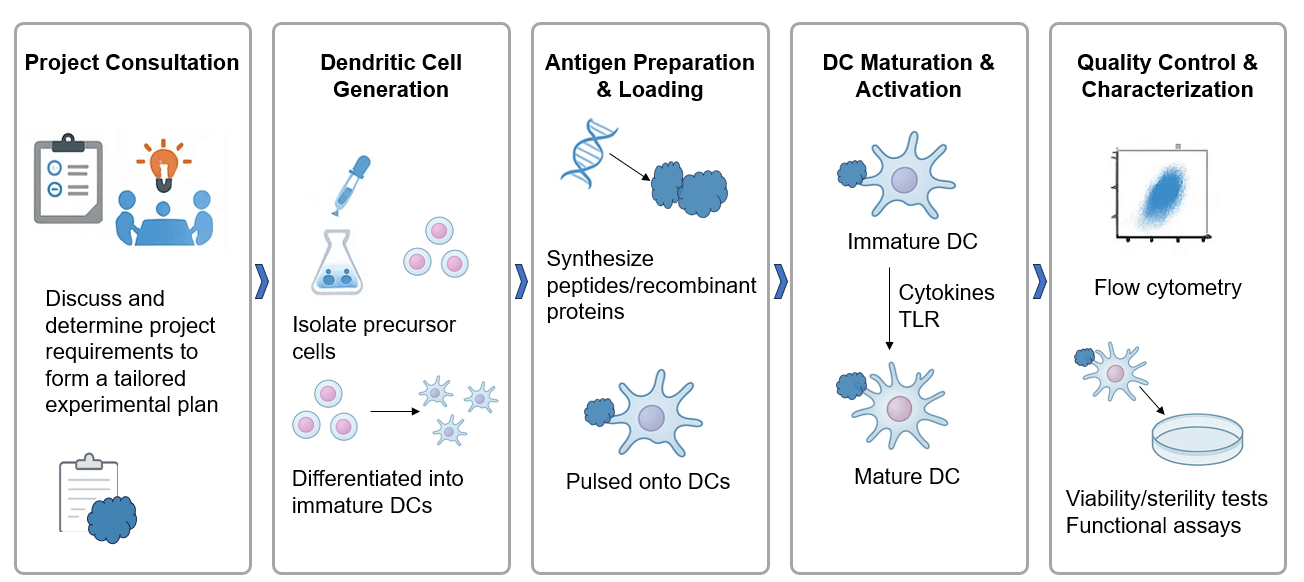
Dendritic Cell Sourcing and Generation
Antigen Design, Synthesis, and Sourcing
Precision Antigen Loading Techniques
Dendritic Cell Maturation and Activation
Comprehensive Characterization and Functional Analysis
Q1: What types of antigens can be used for pulsing DCs with Creative Biolabs?
A1: We offer versatile solutions. Our service can precisely load DCs with various antigen types, including synthetic peptides (e.g., tumor-associated antigen epitopes, personalized neoantigens), full-length recombinant proteins, and even mRNA encoding your target antigens. This flexibility ensures comprehensive epitope presentation.
Q2: How does Creative Biolabs ensure the quality and functionality of the pulsed DCs?
A2: We employ a multi-faceted quality control approach. This includes extensive phenotypic profiling using flow cytometry to confirm DC maturation markers, viability assessment, sterility testing, and robust functional assays such as cytokine secretion and antigen-specific T-cell activation assays. This thorough characterization guarantees the potency and safety of our DC products.
Q3: Is your service suitable for developing personalized cancer vaccines?
A3: Absolutely. Our service is ideally suited for personalized cancer vaccine development, particularly through our expertise in neoantigen identification via advanced bioinformatics (whole-exome and RNA sequencing) and subsequent precision peptide loading. We can help you generate highly specific, patient-tailored DC vaccines.
Choosing Creative Biolabs for your antigen-pulsed DC development service means partnering with a leader dedicated to scientific excellence and client success. Our unique advantages stem from decades of specialized experience, state-of-the-art technology, and an unwavering commitment to quality.
"We previously struggled with consistent DC maturation and loading. Creative Biolabs' service provided incredibly reliable, high-quality DCs, facilitating our vaccine development and ensuring reproducibility across experiments. The detailed characterization reports are invaluable." — Dr. J. Bro.
"For our neoantigen vaccine program, Creative Biolabs' expertise in precision peptide loading was critical. They meticulously synthesized and loaded our patient-specific neoepitopes, resulting in antigen-pulsed DCs that truly elicited the targeted immune responses we needed, far surpassing our previous internal efforts." — Prof. M. Van.
How to Contact Us
Are you set to elevate your immunotherapy project to new heights? Our specialized experts are available to have in-depth conversations about your unique objectives and offer a customized plan, harnessing the power of precision antigen-pulsed dendritic cells.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION