The potential of T cells to recognize and eradicate cancer has fueled research into developing robust methods for generating large quantities of tumor-specific T cells for cancer immunotherapy. Recognizing that the signals received by T cells from antigen-presenting cells during tumor antigen exposure significantly impact their function and therapeutic effectiveness, researchers are actively developing artificial T cell stimulators. These engineered platforms aim to precisely control the signals delivered to T cells, enabling the generation of highly effective and tailored T cell therapies for cancer treatment.
Based on the origin of artificial T cell stimulators, they can be broadly classified into:
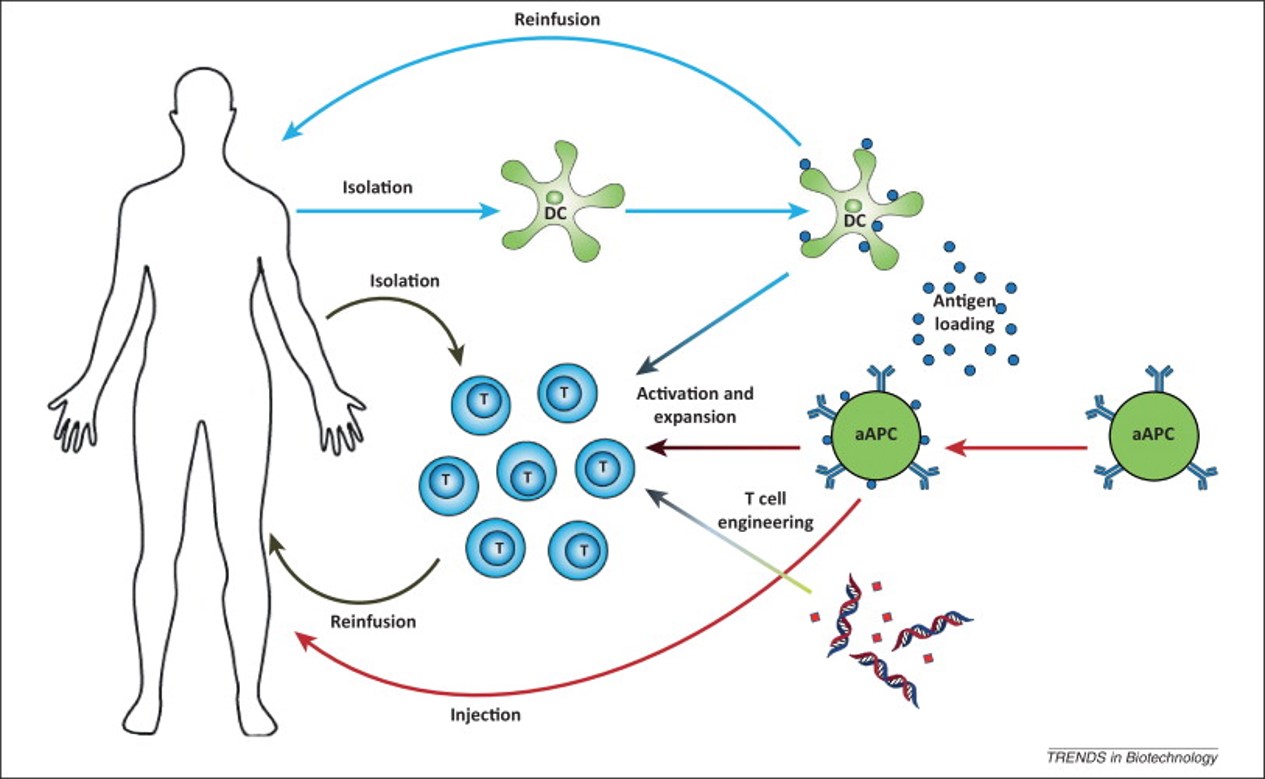 Fig.1 Various active cancer immunotherapy approaches.1,3
Fig.1 Various active cancer immunotherapy approaches.1,3
Creative Biolabs offers a comprehensive artificial T cell stimulator function characterization service to evaluate the safety and efficacy of artificial T cell stimulators. Our services cover the interaction between artificial T cell stimulators and T cells, antigen presentation ability, co-stimulatory molecule expression, cytokine secretion spectrum, and other aspects. Through a series of rigorous in vitro and in vivo experiments, we can accurately quantify the extent to which artificial T cell stimulator induces T cell activation, proliferation, differentiation, and effector function, as well as evaluate its anti-tumor activity in tumor models. In addition, we also provide customized services to meet the different needs of customers in the artificial T cell stimulator development process, aiding customers in accelerating the development of new-generation immunotherapy products.
|
We implement comprehensive analysis of artificial T cell stimulator surface markers, co-stimulatory molecules, and cytokine expression by flow cytometry, immunofluorescence, etc. |
|
We characterize the ability of artificial T cell stimulators to induce T cell activation, proliferation, and differentiation via ELISPOT, flow cytometry, and other methods. |
|
Through cytotoxicity experiments, cytokine detection, and other methods, we can assess the killing activity of T cells, cytokine secretion levels, etc. |
|
We employ animal models to assess the anti-tumor effect of artificial T cell stimulator-induced T cells in vivo. |
Background: NK cells, despite their potent anti-tumor activity, face hurdles in clinical translation. CAR-NK cells show promise, but limitations include suboptimal in vitro expansion, low frequencies in peripheral blood/cord blood, and challenges in achieving consistent and robust therapeutic responses.
Summary: This study engineered HLA-deficient K562 cells to express CD48, 4-1BBL, and mbIL21, creating a universal T cell stimulator that potently stimulates NK cell expansion. The results reveal that co-culture with universal T cell stimulators significantly expanded both non-transduced and CAR-transduced cord blood-derived NK cells with high purity and without signs of exhaustion. Importantly, universal T cell stimulator-expanded NK cells exhibited enhanced metabolic fitness and superior antitumor activity compared to conventionally cultured counterparts.
Phenotypic Characterization
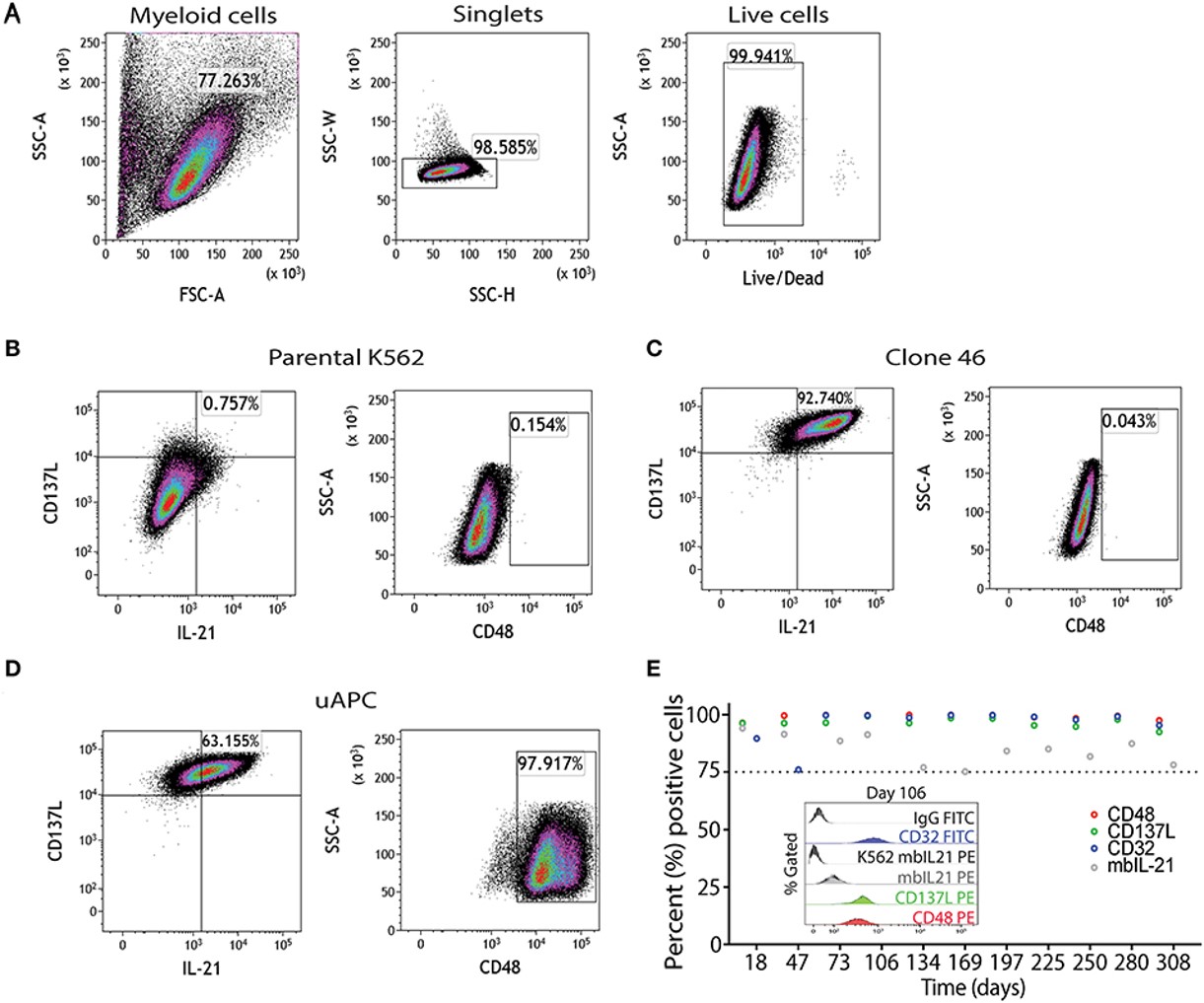 Fig.2 Universal T cell stimulator phenotyping.2,4
Fig.2 Universal T cell stimulator phenotyping.2,4
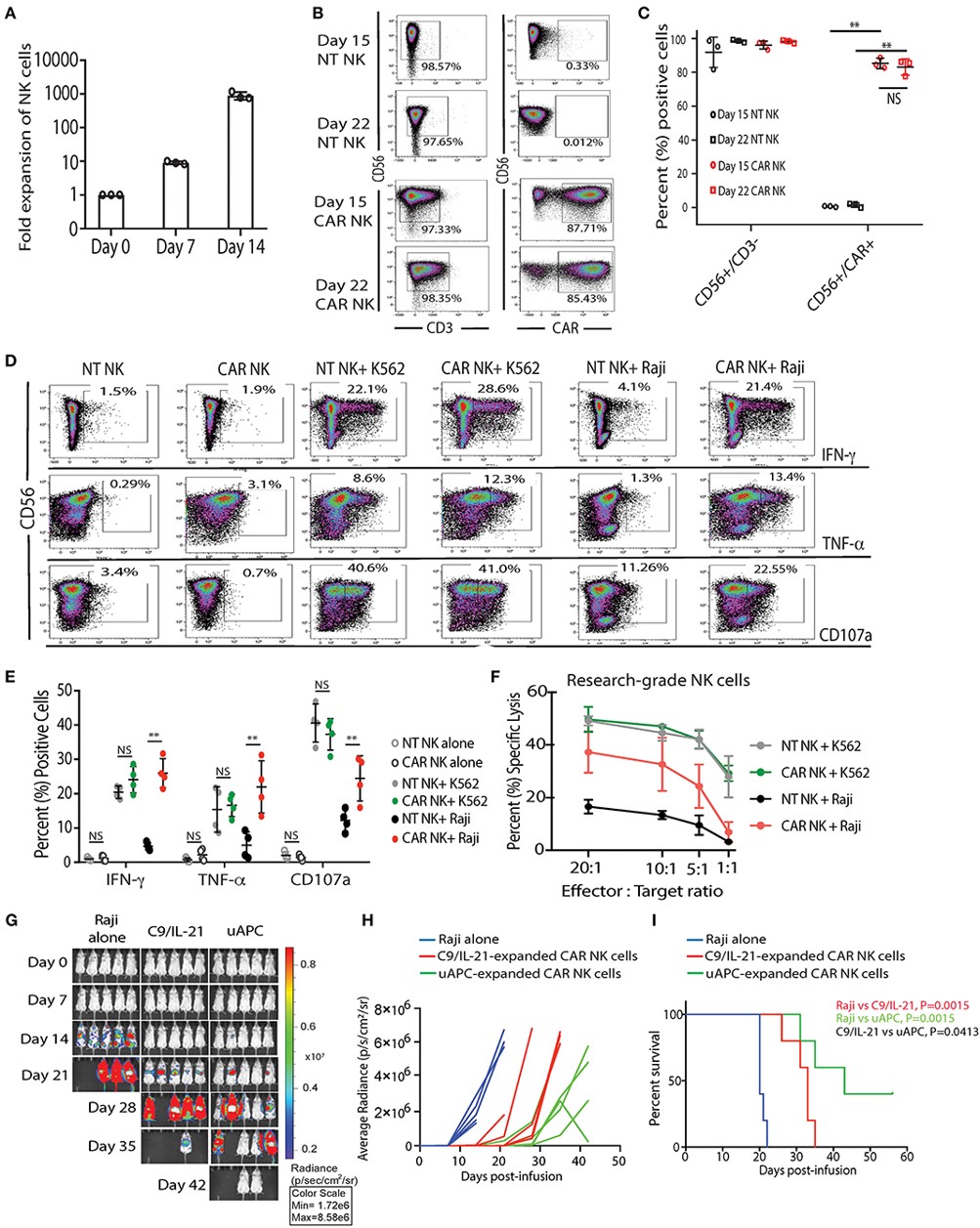 Fig.3 The expansion and cytotoxicity of research-grade NT and iC9/CAR19/IL-15 NK cells are stimulated by universal T cell stimulator.2,4
Fig.3 The expansion and cytotoxicity of research-grade NT and iC9/CAR19/IL-15 NK cells are stimulated by universal T cell stimulator.2,4
If you are interested in our comprehensive artificial T cell stimulator function characterization service, please feel free to get in touch with us.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION