Non-human leukocyte antigen (HLA) antibody specific for angiotensin II type 1 receptor (AT1R) of vascular cells activates signaling pathways, leading to cell proliferation and vascular injury, which can be involved in vascular allograft rejection. Creative Biolabs offers a new approach, AT1R assay, for transplant research at a non-HLA level.
Expressed on the surface of endothelial cells, AT1R combines with angiotensin II to regulate water-salt balance and blood pressure. It belongs to the rhodopsin group (family A) which is one of G protein-coupled receptors composed of seven transmembrane domains. AT1R antibody is the most widely studied non-HLA antibody. They are known to act directly on endothelial cells and smooth muscle cells, consequently activating transcription factors associated with inflammatory reactions and damaging the transplanted kidney. The detection of biomarker antibodies against AT1R offers a new approach to transplant diagnostic testing at a non-HLA level.
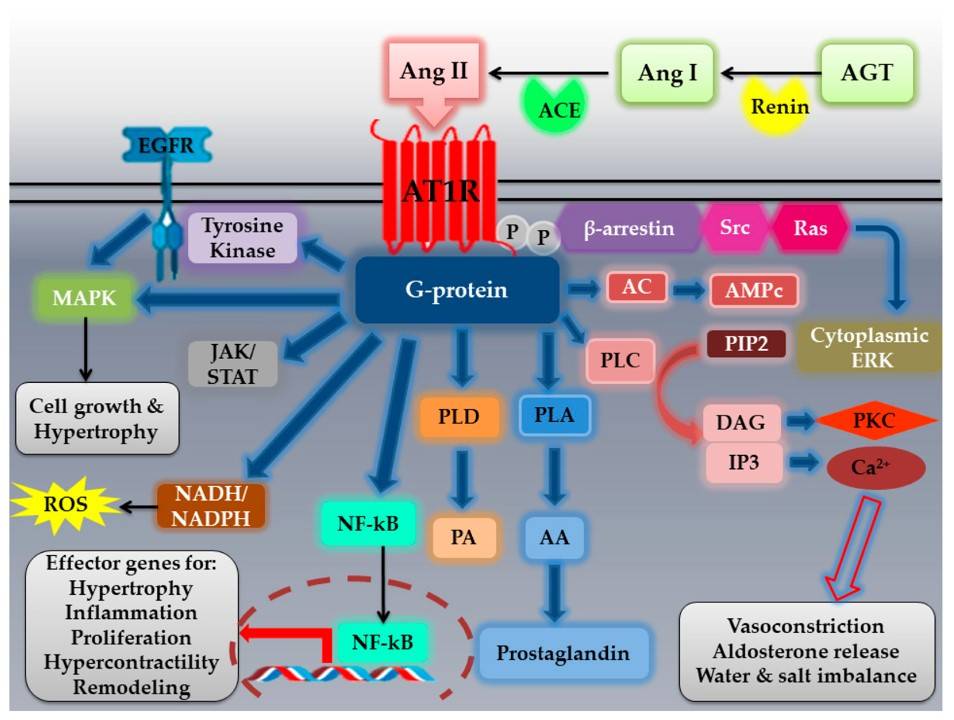 Fig.1 Angiotensin II type I receptor (AT1R) activation by angiotensin II (Ang II) and its signaling pathways.1
Fig.1 Angiotensin II type I receptor (AT1R) activation by angiotensin II (Ang II) and its signaling pathways.1
High levels of anti-AT1R antibody are associated with morphological and functional allograft injury and graft loss in renal transplant patients. This means that non-HLA antibodies can help assess the risk of graft failure.
AT1R antibodies may identify kidney transplant recipients at high risk of allograft rejection and loss, independent of the HLA system. Recognition of complement-independent AT1R antibody-mediated vascular rejection could lead to the development of new treatment strategies to improve allograft survival.
Heart transplantation recipients with diagnosed cardiac allograft vasculopathy (CAV) possess a high frequency for positive detection of AT1R antibodies. Additionally, non-HLA antibodies are linked to the earlier incidence of CAV after heart transplantation. The screening of non-HLA antibodies after heart transplantation is recommended to identify patients with an increased risk for CAV.
 Fig.2 The workflow of AT1R assay.
Fig.2 The workflow of AT1R assay.
Creative Biolabs offers AT1R assay for the convenience of our clients. As a world-famous antibody provider, we also provide high-quality AT1R antibody development services. For more information, please feel free to contact us directly.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION