Are you currently facing challenges related to high data variability, complex target preparation, or low throughput in evaluating antibody-dependent cellular phagocytosis (ADCP) or the function of macrophages? Creative Biolabs' Bead Engulfment Assay based Phagocytosis Evaluation Service helps you obtain highly reproducible, quantitative functional data through an advanced, flow cytometry-based microparticle platform, significantly reducing the complexity associated with native cell- or pathogen-based assays. The standardized results we delivered are crucial for therapeutic and vaccine development.
Phagocytosis, the process by which immune cells engulf and clear cellular debris, pathogens, and opsonized targets, is a critical component of the innate and adaptive immune response. Traditional methods often suffer from high variability and low throughput, especially when using complex or difficult-to-culture biological targets. The bead engulfment assay (BEA) overcomes these limitations by utilizing standardized, functionalized fluorescent microparticles as stable, uniform surrogates. This flow-cytometry-based approach enables rapid, quantitative, and highly reproducible measurement of ADCP efficacy in therapeutic and vaccine development.
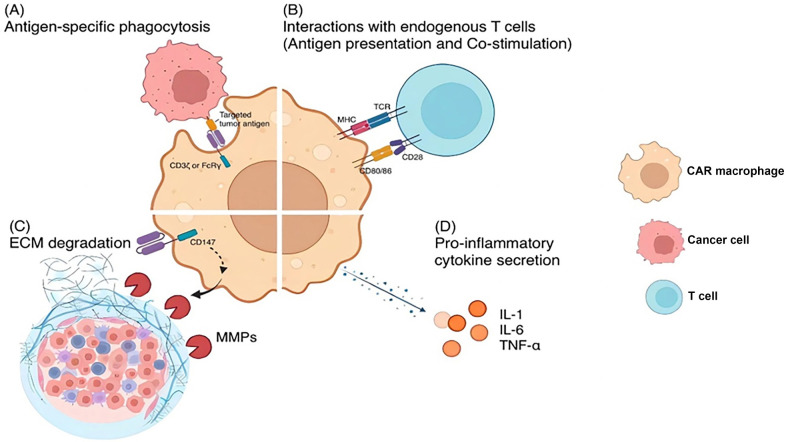 Fig.1 Strategies for macrophage-mediated tumor cell phagocytosis.1
Fig.1 Strategies for macrophage-mediated tumor cell phagocytosis.1
Creative Biolabs' Bead Engulfment Assay based Phagocytosis Evaluation Service is designed to precisely measure the functional capacity of therapeutic antibodies or plasma samples to induce phagocytosis. By employing standardized, functionalized fluorescent beads as surrogate targets, we eliminate the high biological variability inherent in using native infected cells or complex primary cell lines. We provide quantitative, reproducible data essential for advancing drug candidates and validating vaccine efficacy in high-throughput formats.
We provide precise phagocyte functional analysis by employing standardized, quantifiable beads that simulate physiologically relevant targets, which enables unbiased quantification of phagocytic activity in key immune cells. Our core technical strengths lie in:
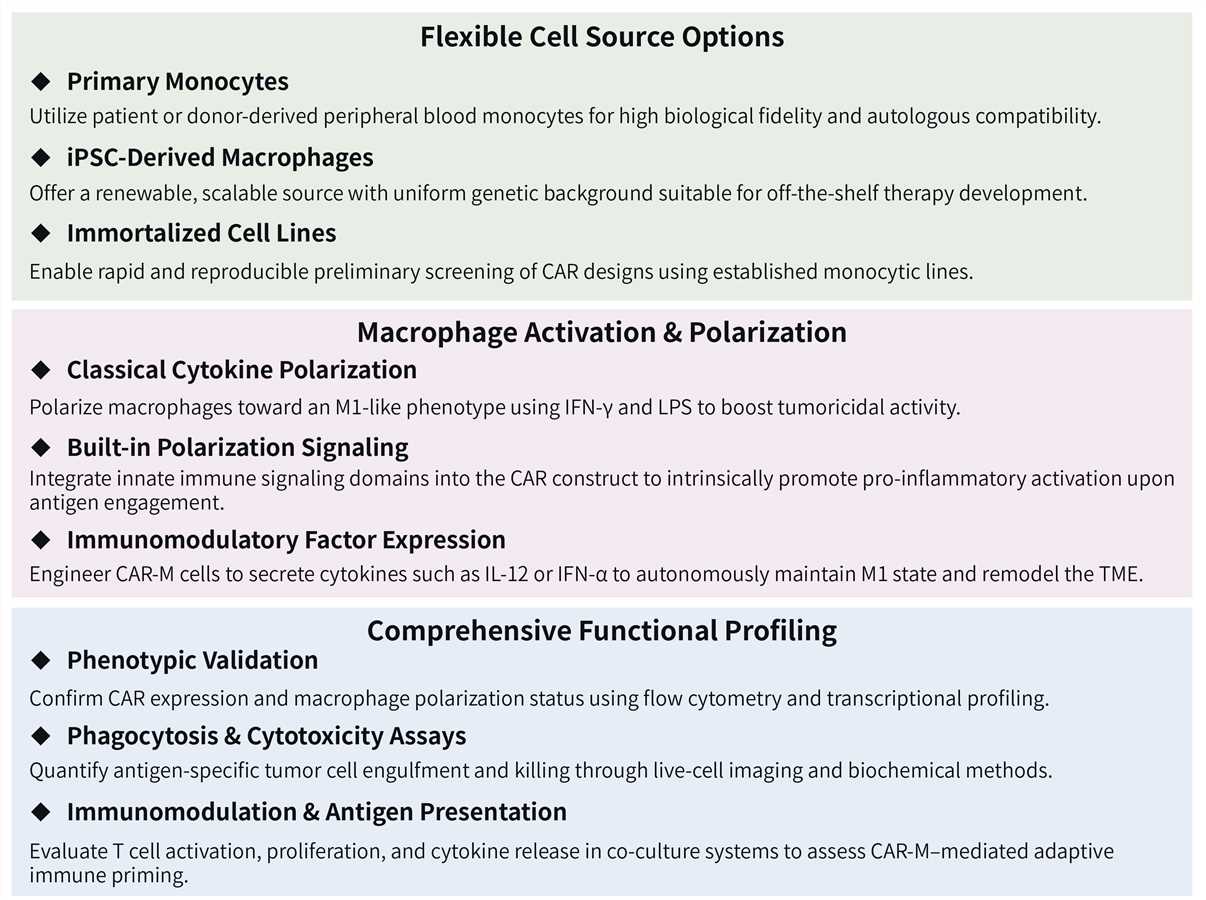
In addition to our diverse range of phagocytosis assays, which utilize targets such as bacteria, beads, and tumor cells, we also provide specialized CAR-MA-mediated Trogocytosis Assay and Eating Assay services to support your research.
Required starting materials:
Key Steps Involved:
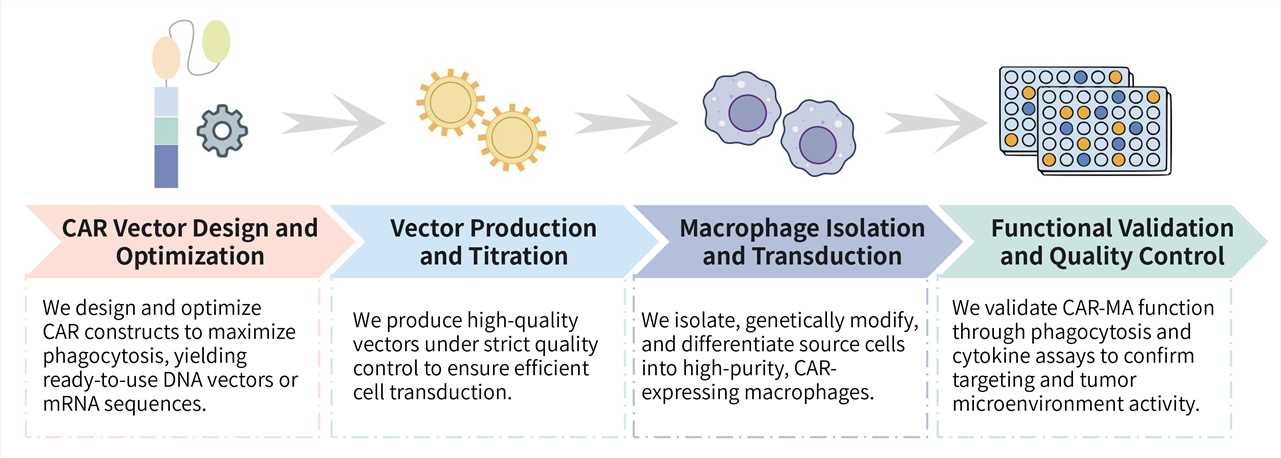
Final Deliverables:
How do you ensure the assay accurately differentiates between surface-bound and truly internalized beads?
We employ a validated two-color flow cytometry gating strategy. After the phagocytosis reaction, we use a secondary antibody conjugated to a fluorophore that only binds to the external (surface-attached) opsonizing antibodies. Internalized beads, protected within the phagosome, remain unstained by the secondary antibody, allowing for definitive gating and quantitative assessment of true engulfment.
Is this assay suitable for high-throughput screening of large antibody libraries?
Yes, our bead engulfment assay is designed specifically for high-throughput screening. The use of robust, standardized beads and a rapid, plate-reader-equipped flow cytometer allows for the efficient processing of hundreds of samples daily, making it ideal for large-scale therapeutic antibody or clinical sample screening projects.
Creative Biolabs accelerates your therapeutic development with our reliable, quantitative Bead Engulfment Assay. Our standardized, flow cytometry-optimized platform minimizes experimental variability, delivering the robust, actionable data you need to streamline pipeline decisions.
"Using Creative Biolabs' Bead Engulfment Assay based Phagocytosis Evaluation Service in our research has significantly improved the reproducibility of our ADCP data for new IgG isotypes, allowing us to confidently move our lead molecules forward."— Dr. N***n R***e.
"The ability to reliably quantify phagocytosis in primary microglial cultures using this service was a game-changer. We finally have a high-throughput method that gives us clear, quantitative data on CNS immune cell function, which was previously a major bottleneck."— E***y M***s.
"We needed to screen hundreds of patient plasma samples for opsonic activity against a recombinant pathogen antigen. Creative Biolabs delivered a highly repeatable, flow-optimized solution that allowed us to efficiently process the entire cohort in a fraction of the time compared to using traditional methods."— Jl Fr.
Ready to standardize your phagocytosis assessment? Reach out to our specialists today to review your project needs, obtain a comprehensive quotation, and explore how our dedicated bead assay services can enhance your upcoming research.
Contact Our Team to Learn More and Advance Your Project.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION