Chimeric Antigen Receptor T-cell (CAR-T) therapy has revolutionized the field of cancer treatment by leveraging the patient's immune cells to abate malignancies. This innovative treatment involves reprogramming T cells to express CARs that can specifically target cancer cells. CAR-T therapy has shown remarkable success in treating certain types of leukemia and lymphoma, offering hope to patients who have exhausted conventional treatment options. At Creative Biolabs, we are committed to advancing this promising therapeutic area by providing global Contract Development and Manufacturing Organization (CDMO) services for CAR-T therapies, ensuring high-quality and efficient production processes.
IVT RNA Platform
Our IVT RNA service utilizes advanced IVT technology to produce high-quality RNA quickly and efficiently, ensuring optimal purity and yield for a variety of applications.

LNP Encapsulation Platform
our LNP encapsulation service is designed to optimize the delivery of RNA and other therapeutics. Our state-of-the-art technology ensures efficient encapsulation, enhancing stability and bioavailability while minimizing degradation.
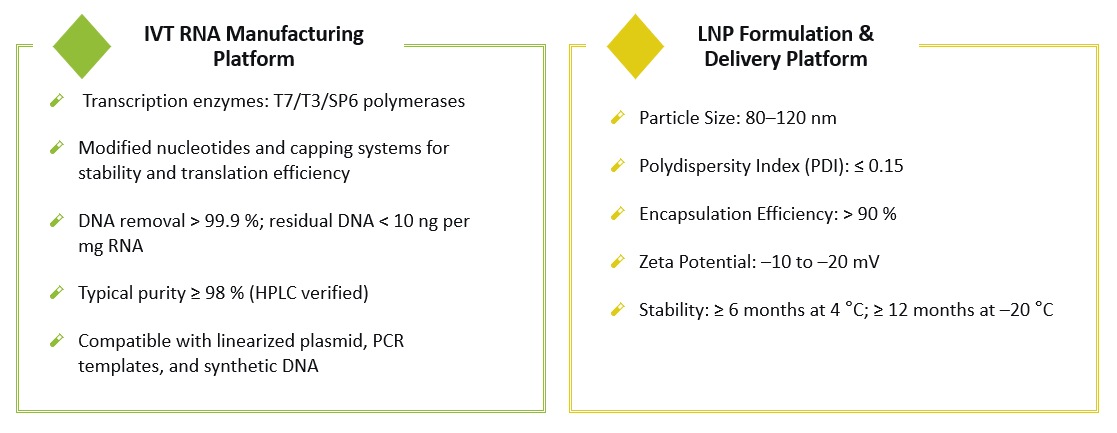
The production of CAR IVT RNA is a critical step in the development of CAR-T therapies. In vitro transcription (IVT) RNA encompasses a suite of synthetic RNA sequence production techniques that are essential for expressing CAR constructs. Our state-of-the-art CAR IVT RNA manufacturing platform at Creative Biolabs is accurately designed to deliver high-purity, high-quality RNA products that cater to various stages of research and clinical development. This platform ensures the scalability and reproducibility of CAR IVT RNA. We employ advanced technologies such as high-fidelity RNA polymerases, optimized transcription conditions, and rigorous purification methods to achieve superior RNA yields and functionality.
Our platform provides various grades of CAR IVT mRNA, ensuring that every project has a tailored solution:
| Research Grade (RG) mRNA | GMP-like mRNA | GMP Grade mRNA | |
| Purpose | Used for basic research and early-stage development | Suitable for preclinical studies and preliminary clinical investigations | Used for clinical trials and commercial applications |
| Quality Control | Limited QC testing | Intermediate level of QC testing to ensure product consistency and quality | Comprehensive QC testing, including purity, potency, and safety assessments |
| Regulations | Do not conform to GMP standards | Partially conforms to GMP standards, making it more rigorous than research-grade but not fully GMP-compliant | Fully GMP-compliant, adhering to strict regulatory requirements |
| Scale | Typically small-scale production | Scalable production suitable for clinical study needs | Large-scale production to meet clinical and commercial demands |
Lipid Nanoparticle (LNP) technology represents an advanced approach to the delivery of RNA therapeutics. LNPs enhance the stability and delivery efficiency of therapeutic RNAs, ensuring their successful transport into target cells. At Creative Biolabs, our CAR-LNP platform integrates cutting-edge techniques to formulate and characterize LNP-encapsulated CAR RNAs. Our platform focuses on key aspects such as lipid composition optimization, particle size control, encapsulation efficiency, and in vitro/in vivo delivery performance. We provide comprehensive solutions from formulation development to scale-up manufacturing. This ensures that the CAR RNA payloads are protected from degradation and delivered precisely to the intended cells, maximizing therapeutic efficacy.
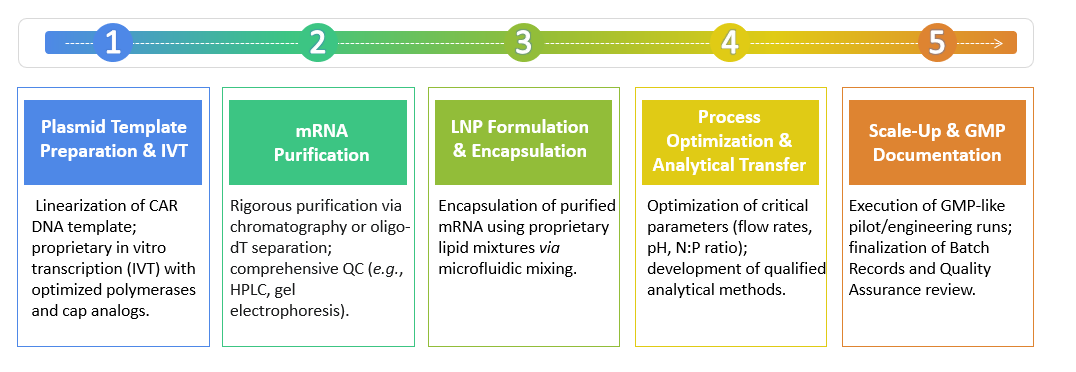
At Creative Biolabs, our workflow for CAR IVT RNA and LNP manufacturing services begins with the design and synthesis of the CAR construct, followed by the in vitro transcription to produce high-fidelity CAR RNA. This RNA is then subjected to rigorous purification protocols to remove impurities. Concurrently, our LNP formulation specialists develop and optimize LNP carriers tailored for the specific CAR RNA payloads. The encapsulation process involves incorporating the purified CAR RNA into LNPs under controlled conditions to achieve the desired encapsulation efficiency and particle size. Once the formulation is complete, the CAR-LNP product undergoes extensive characterization to evaluate parameters such as particle size distribution, particle concentration, encapsulation efficiency, payload analysis, and stability. We employ advanced analytical techniques to ensure the consistent quality and functionality of the CAR-LNPs.
The transition from a promising CAR construct to a clinical-grade cellular therapeutic demands an assured supply of vectors that meet the highest standards of safety and potency. Creative Biolabs specializes in de-risking this critical step by providing custom-engineered, cGMP-compliant lentiviral and retroviral vectors, tailored precisely for your T-cell modification requirements.
Each project is supported by a dedicated project manager, ensuring transparent communication, milestone tracking, and on-time delivery.
Every batch of IVT RNA and LNP product is manufactured under a rigorous quality management system and validated analytical framework.
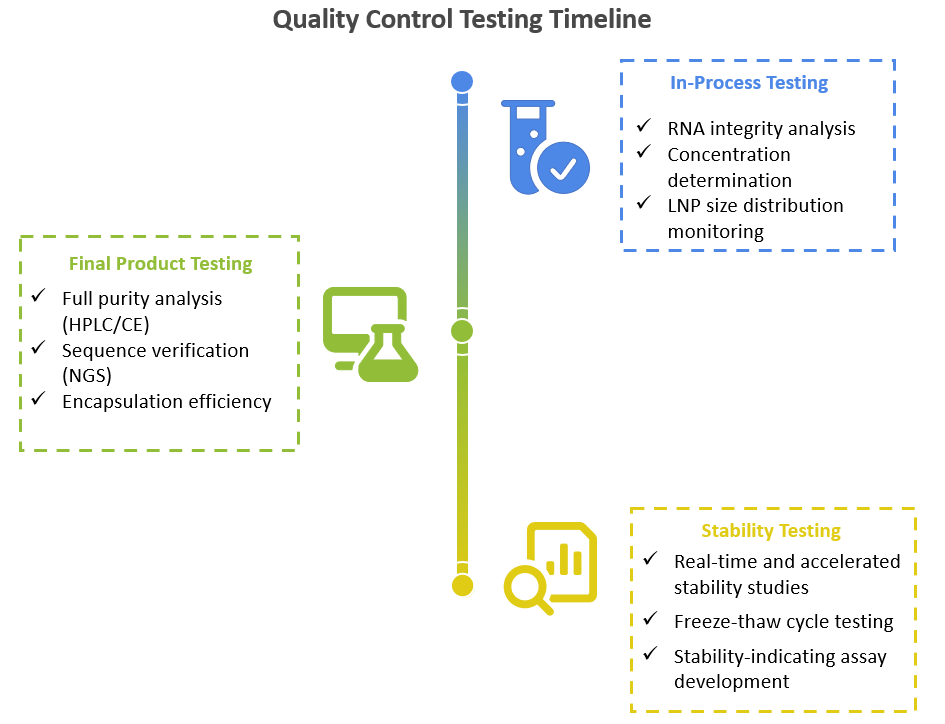
Quality Control Testing Timeline
Experience the Creative Biolabs Advantage - Get a Quote Today
How does LNP encapsulation compare to standard T-cell electroporation, particularly regarding cell viability and transfection consistency?
While electroporation is viable, LNP offers superior clinical performance. LNPs shield cells, resulting in higher T-cell viability. Our proprietary LNP and microfluidic mixing ensure highly consistent transfection efficiency across large batches, mitigating common variability and cell loss. Contact our technical team to review comparative data on T-cell recovery and potency.
What are the primary safety and immunogenicity precautions associated with the LNP components or the transient CAR expression?
Safety is paramount, starting with the elimination of genomic integration risk. We use non-immunogenic, highly biocompatible lipid mixes and rigorous purification to remove dsRNA impurities, minimizing innate immune activation. We provide a complete safety package with all GMP batches. Request a consultation to discuss lipid composition and QC standards for regulatory filing.
Can Creative Biolabs' LNP platform be efficiently utilized to modify other immune cells besides primary T-cells, such as NK cells or macrophages?
Absolutely. The modular nature of LNP formulation allows us to engineer a wide range of immune cells, including CAR-NK cells and CAR-Macrophages. Our Custom LNP Formulation services tailor lipid ratios and components to achieve optimal uptake and transfection efficiency in your specific target cell population. Let us know your cell type so we can provide a targeted formulation strategy.
Using Creative Biolabs' CAR IVT RNA and LNP Manufacturing Services in our research has significantly improved the tolerability profile of our transiently modified cells. The purification process for their mRNA clearly minimizes dsRNA, resulting in T-cells that show markedly reduced inflammatory cytokine release compared to our in-house mRNA prep. (Dr. A***ers)
The GMP-like master batch record and CoA data provided by Creative Biolabs were essential for our preliminary filing. Their focus on particle size distribution consistency across different scales ensured a smooth transition from preclinical to Phase I manufacturing, avoiding costly process re-development. (J. L***g)
Contact Our Team for More Information and to Discuss Your Project.
Our integrated workflow ensures a seamless and streamlined process from initial design to final production, providing our clients with reliable and robust manufacturing solutions for their CAR-T therapeutic development needs. Creative Biolabs is dedicated to supporting the advancement of CAR-T therapies, offering expertise and state-of-the-art technologies to propel groundbreaking cancer treatments forward. Contact us for more about our IVT RNA and LNP manufacturing services.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION