In CAR-M therapy, the strategic secretion of a broad spectrum of cytokines is central to orchestrating a multifaceted antitumor response, directly mediating efficacy by recruiting adaptive immune cells and reshaping the immunosuppressive tumor microenvironment. Creative Biolabs' CAR-MA Cytokine Release Assay Service employs advanced multiplex immunoassays to deliver precise quantification of this critical functional output. Our service advantage lies in customized profiling panels, which ensure high sensitivity, specificity, and throughput for uncompromising data quality tailored to specific research and development objectives. We ultimately empower your program by delivering actionable insights for candidate selection.
As pivotal sentinels of the innate immune system, macrophages are a primary source of diverse cytokines, orchestrating both inflammatory and regulatory immune responses. In the context of chimeric antigen receptor macrophage (CAR-MA) therapy, this inherent capability is strategically harnessed, where upon tumor antigen recognition, engineered CAR-MA cells secrete a spectrum of inflammatory cytokines that directly mediate antitumor efficacy by recruiting and activating adaptive immune cells and remodeling the immunosuppressive tumor microenvironment. Consequently, the quantitative detection of cytokine release is indispensable for the functional validation, potency assessment, and translational development of CAR-MA therapeutics, serving as a critical biomarker for their activation status and mechanistic depth.
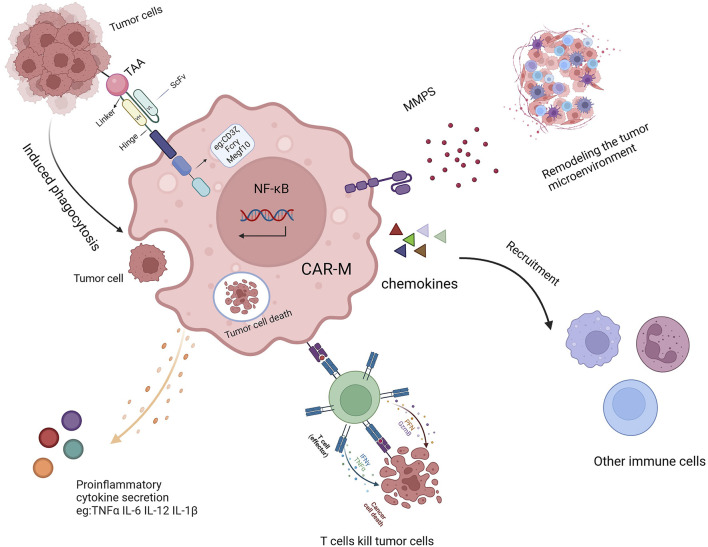 Fig.1 Multimodal mechanism of action of CAR-Macrophages.1
Fig.1 Multimodal mechanism of action of CAR-Macrophages.1
Creative Biolabs' CAR-MA Cytokine Release Assay Service employ robust, quantitative immunoassays to accurately measure immune cell activity, providing crucial functional data for candidate assessment. Our scientists work in close partnership with you, from mechanism of action studies to preclinical evaluation, to establish the most relevant and informative testing protocol for your therapeutic molecule, ensuring robust data generation at every stage.
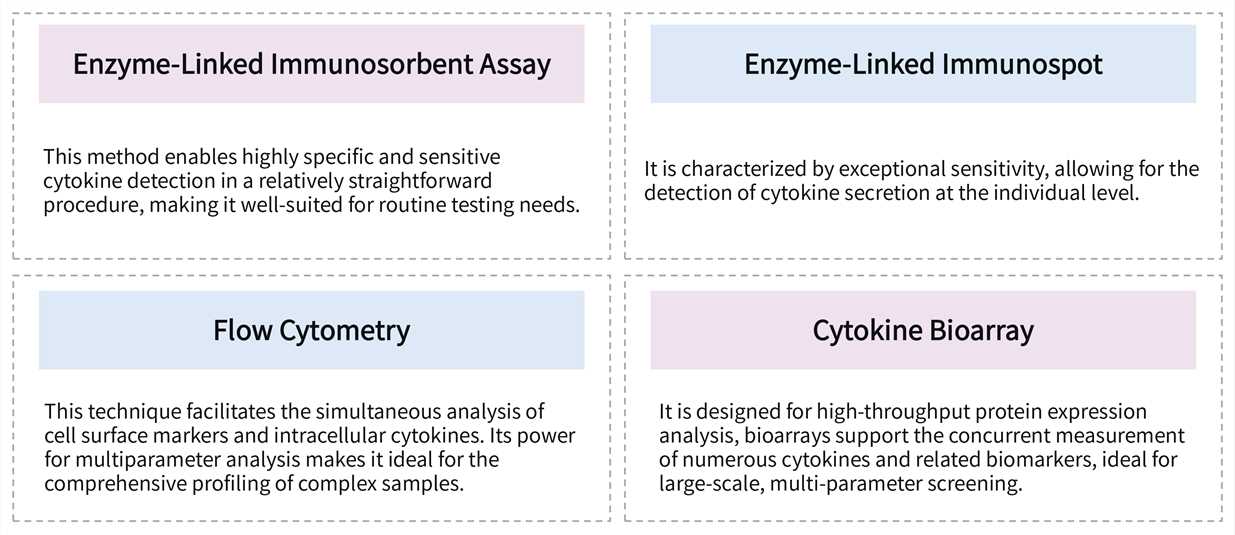
We offer a suite of sophisticated detection methods designed to address diverse experimental needs. Our customizable cytokine detection panels provide high sensitivity, specificity, and throughput, ensuring reliable data quality for your specific research objectives.
Required starting materials:
Key Steps Involved:
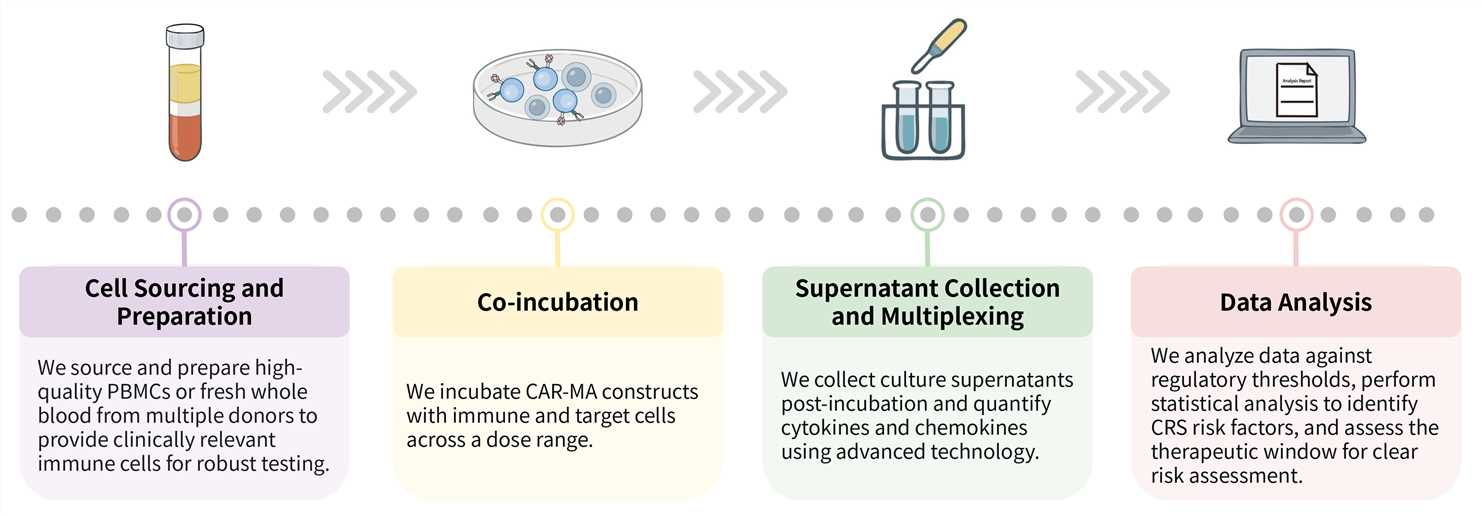
Final Deliverables:
Why is a specialized CAR-MA cytokine release assay required when generic CRS assays exist?
CAR-MA is a unique modality with different kinetics than CAR-T. Standard assays often miss the macrophage-specific cytokine signatures that mediate tumor penetration and T cell attraction. Our specialized service uses specific stimulation protocols and multiplex panels optimized to capture these unique CAR-MA functional and safety endpoints.
Can you perform cytokine release assay on therapeutic modalities other than CAR-MA?
Absolutely. While we specialize in CAR-MA, our advanced multiplexed platforms and validated protocols are fully equipped to assess the CRS risk for all biologics, such as monoclonal antibodies, CAR-T, and CAR-NK therapies.
Leveraging comprehensive expertise in advanced cellular platforms and immunotoxicology, Creative Biolabs provides core empowerment for your CAR-MA program. Our CAR-MA Cytokine Release Assay precisely quantifies the therapeutic activity and inflammatory potential of candidate constructs by integrating physiologically relevant cell models and advanced detection technologies. This delivers critical insights into developability and supports de-risking your therapeutic strategy.
"Using Creative Biolabs' CAR-MA Cytokine Release Assay Service in our research has significantly improved the confidence in our lead selection. Their ability to simultaneously profile the core regulatory panel alongside T cell-specific cytokines allowed us to clearly differentiate our low-risk CAR-MA from a cytotoxic CAR-T benchmark."— A**am P.
"The formal report provided by Creative Biolabs was a standout deliverable. It clearly addressed our concerns regarding cytokine storm risk, particularly the donor variability and dose-response data, streamlining our submission process considerably."— E**a T.
Confidently advance your novel CAR-MA therapy by addressing the critical translational bottleneck of CRS. Creative Biolabs provides a specialized CAR-MA Cytokine Release Assay, leveraging cutting-edge multiplex technology and regulatory insights to precisely quantify CRS risk and support the development of a robust preclinical data package. Connect with our team to explore how we can accelerate your immunotherapy's development.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION