As a pioneering advancement in cellular immunotherapy, chimeric antigen receptor macrophages (CAR-MA) have emerged as a key strategy to overcome the challenges of solid tumor treatment, owing to their unique tumor infiltration capacity, diverse antitumor mechanisms, and ability to reprogram the immunosuppressive tumor microenvironment. Creative Biolabs' CAR-MA Design and Construction Service provides comprehensive technical support spanning from antigen-binding domain optimization and intracellular signaling domain selection to the establishment of viral transduction systems. Our goal is to deliver highly efficient and target-specific CAR-MA research tools and therapeutic candidates, ultimately accelerating their preclinical validation and translational applications in solid tumor immunotherapy.
A pivotal frontier in solid tumor immunotherapy involves overcoming the limitations of CAR-T cells, particularly their susceptibility to the immunosuppressive TME and inefficient tumor trafficking. CAR-MA therapy arises as a promising alternative, capitalizing on the unique abilities of macrophages to penetrate tumors, engage in antigen presentation, and remodel the immunosuppressive niche. Mastering the design and construction of CAR-MA, through tailored signaling domains, optimal cell sourcing, and rational integration of immunomodulatory elements, is therefore central to realizing the potential of this next-generation therapeutic platform.
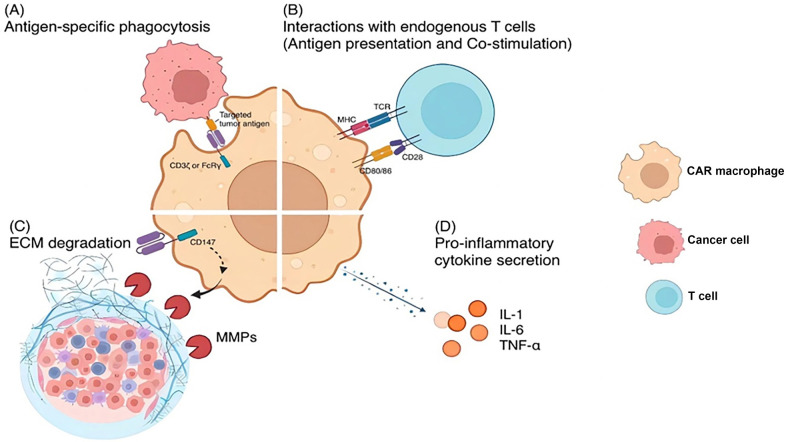 Fig.1 Orchestrating antitumor immunity: the mechanisms of engineered CAR-Macrophages.1
Fig.1 Orchestrating antitumor immunity: the mechanisms of engineered CAR-Macrophages.1
Creative Biolabs' CAR-MA Design and Construction Service is specifically designed to overcome the intrinsic limitations of T cell therapies within the TME. Our approach transcends conventional genetic modification by integrating functional polarization and multi-modal engineering, thereby ensuring superior tumor infiltration and sustained anti-tumor activity. We deliver not only advanced cell candidates but a full strategic partnership to accelerate your therapy from concept to clinic.
Our CAR-MA design and construction service is powered by a flexible and advanced platform to ensure your candidate is seamlessly optimized for efficacy and clinical translation.
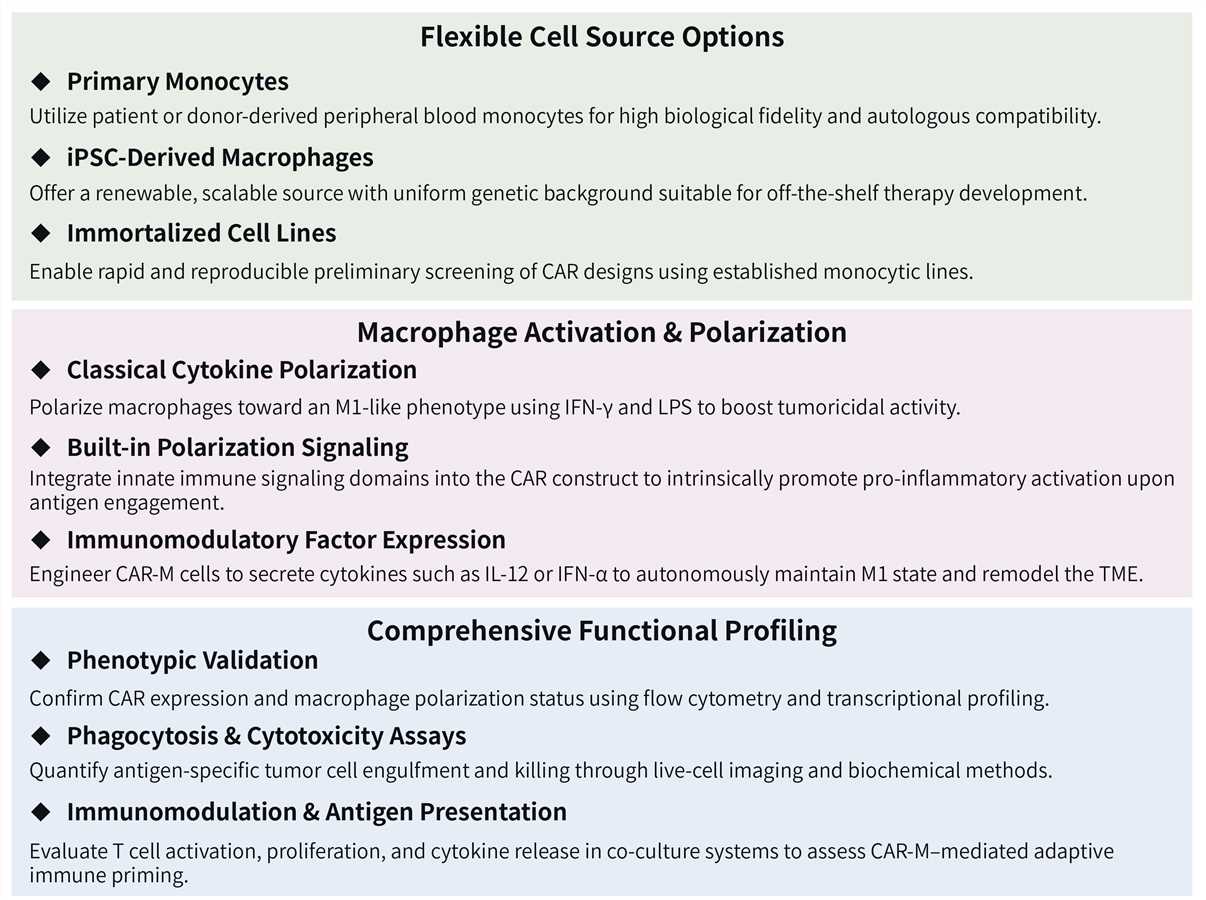
Required starting materials:
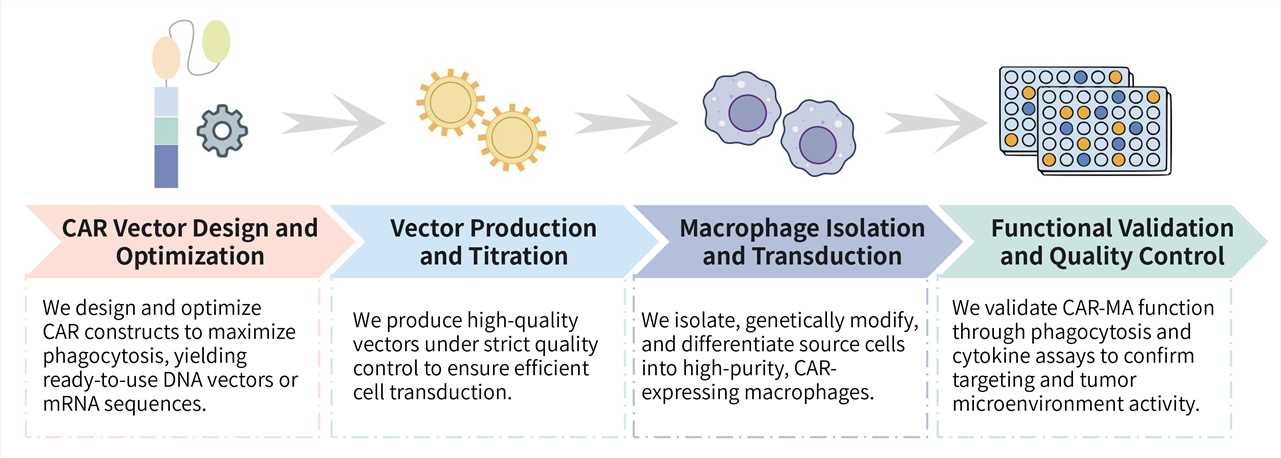
Key Steps Involved:
Final Deliverables:
How does the CAR-MA safety profile compare to the risk of CRS associated with CAR-T?
CAR-MA cells are generally observed to have a more favorable safety profile. Unlike the intense, systemic cytokine burst often seen with CAR-T, CAR-M typically exhibits different cytokine release kinetics, potentially leading to less severe CRS and neurotoxicity. Our design process prioritizes functional phagocytosis over excessive pro-inflammatory cytokine production to maintain this safety advantage.
Which macrophage cell source (iPSC, Primary Monocyte, or Cell Line) do you recommend, and why?
The optimal source depends entirely on your therapeutic goal. Primary monocyte-derived CAR-MA offers high fidelity but low scalability. iMacs are ideal for large-scale, allogeneic, off-the-shelf production. Cell lines offer speed for early-stage screening. We provide detailed consultation to help you choose the best source.
Our CAR-MA design and construction service is built upon a deep understanding of both the challenges in solid tumor immunotherapy and the latest scientific breakthroughs. we engineer CAR-MA constructs with customizable features to not only directly eliminate tumor cells but also recruit and activate endogenous T and NK cells, turning the tumor into a site of coordinated immune attack.
"Using Creative Biolabs' CAR-MA Design and Construction Service in our research has significantly improved the transduction efficiency in our primary monocyte-derived macrophages, consistently achieving over 85% CAR expression, which was critical for moving into in vivo studies. Their vector design was clearly superior to the off-the-shelf options we previously tested."— Dr. Je P*r.
"The functional data received from Creative Biolabs on cytokine release profiling was invaluable. It demonstrated a pronounced M1 polarization shift in our CAR-MA, confirming its capacity to actively reprogram the immunosuppressive tumor microenvironment, which is a major advantage over previous CAR-T attempts."— Ms. My C*z.
"Creative Biolabs successfully delivered a highly pure, iPSC-derived CAR-MA product. Their ability to handle the entire differentiation, transduction, and quality control process allowed us to save nearly six months on our preclinical timeline. The documentation for the final product was exceptional."—Dr. An R*o.
To leverage our CAR-MA design and construction capabilities and expedite your therapeutic pipeline, we invite you to connect with our Lead Scientific Officers for dedicated scientific support.
Leverage our scientific expertise to accelerate your development timeline.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION