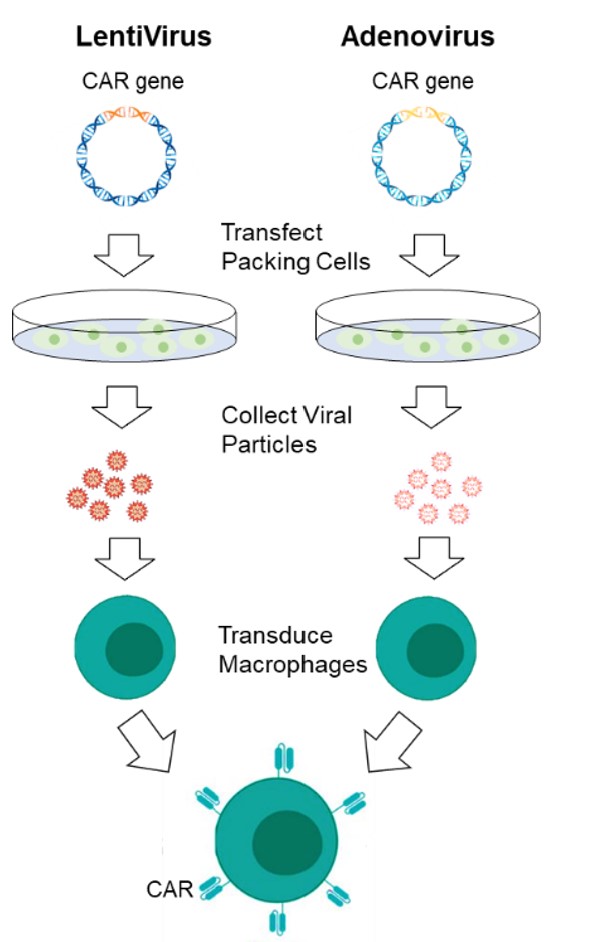
 Fig.1 CAR-MA transfection approaches.
Fig.1 CAR-MA transfection approaches.
(Creative Biolabs)
Chimeric antigen receptor (CAR) T cell therapy has shown promise in hematologic malignancies, but its application to solid tumors has been challenging. Given the unique effector functions of macrophages and their capacity to penetrate tumors, genetically engineered human macrophages with CARs make it possible to produce direct phagocytic activity against tumors. Creative Biolabs is pleased to announce our custom chimeric antigen receptor macrophage (CAR-MA) preparation service.
Macrophages are highly resistant to genetic engineering with standard vectors such as lentivirus, retrovirus, and adeno-associated viruses (AAV). The vector requires fine-tuning and molecular adjustments to better transfer to macrophages.
Creative Biolabs provides reliable and available sources of macrophages including induced pluripotent stem cells (iPSC), human monocytic cell line (THP-1), human primary macrophages (CD14+ peripheral blood), murine macrophages (Raw264.7, J774A.1), etc. In general, iPSC-derived macrophages may become an important source of cells for myeloid-based cancer immunotherapy.
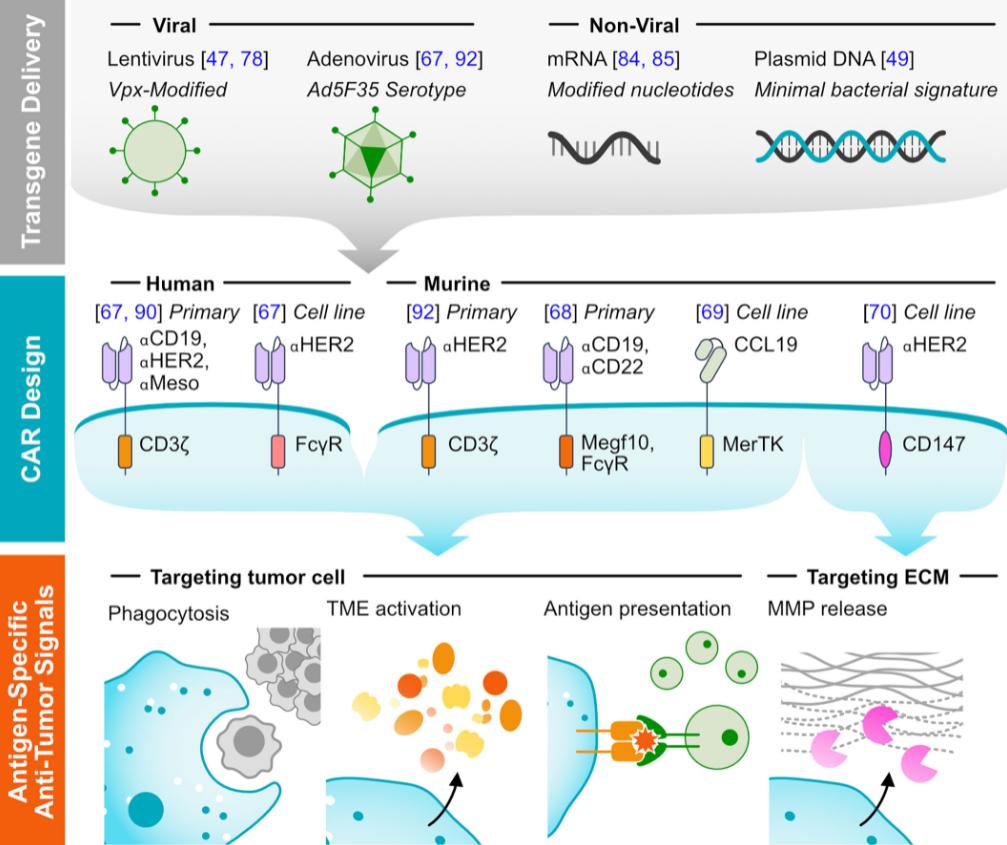 Fig. 2 CAR-MA generation.1
Fig. 2 CAR-MA generation.1
With an experienced expert team, Creative Biolabs offers high-quality CAR-MA preparation services. If you are interested in our services, please feel free to contact us.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION