Innovative genetic engineering and gene therapy approaches have transformed the landscape of medical research and clinical applications. A key component in these advanced therapies is the delivery system, where viral vectors play a crucial role. As a front-runner in this field, Creative Biolabs offers comprehensive CAR viral vector CDMO services, including both AAV packaging and lentivirus packaging, tailored to meet diverse research and therapeutic needs.

Comprehensive GMP AAV experience

Optimized packaging protocols

Customizable solutions

Strict in-house QC
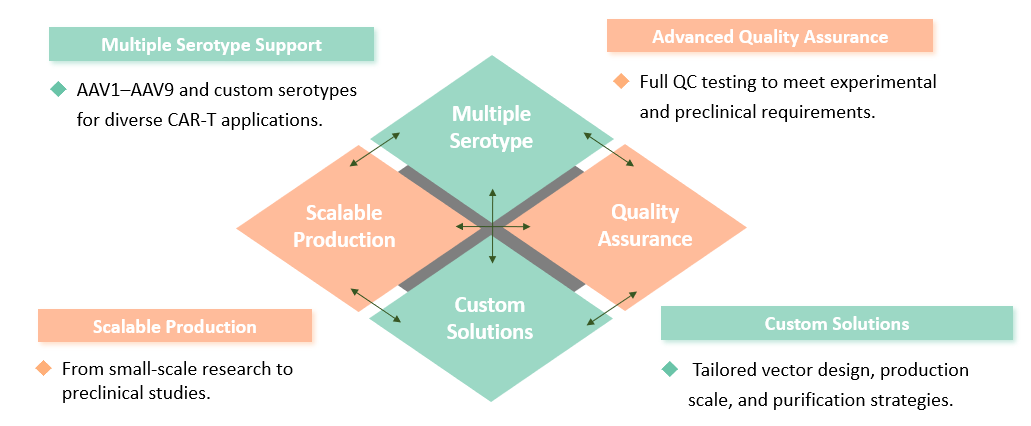
At Creative Biolabs, our AAV packaging platform is designed to offer superior packaging services tailored specifically for CAR-T cell therapy. Leveraging years of expertise in gene therapy, we provide comprehensive solutions from vector design to production, ensuring the highest levels of efficacy and safety. We employ a scalable production process that accommodates various serotypes and payloads, allowing for versatility in addressing a wide range of therapeutic indications. Our AAV packaging utilizes robust methodologies to generate high-titer, high-purity AAV essential for CAR-T applications.
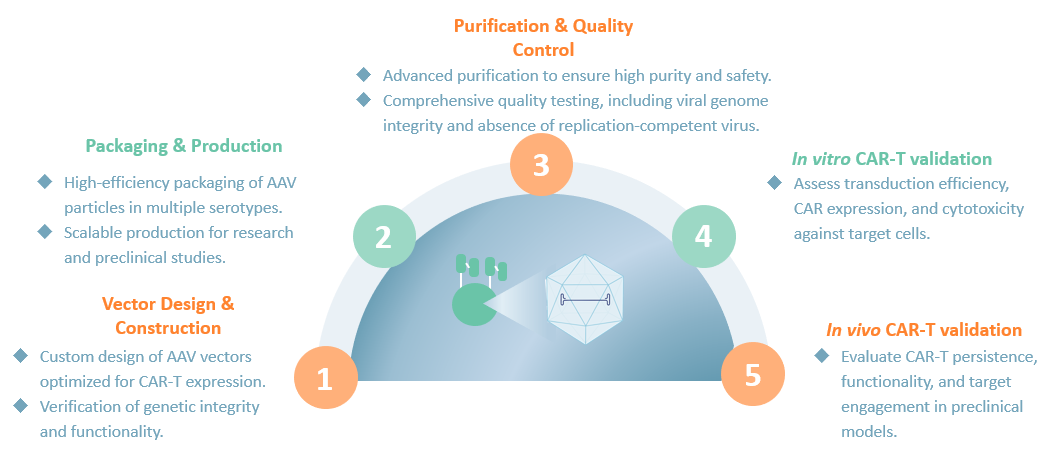
From cell line development to optimized transfection, virus generation, purification, and QC, we deliver a fully integrated AAV production workflow designed for efficiency and precision.
We ensure measurable, reliable performance for all our AAV packages:
| Metric | Standard |
|---|---|
| Viral Titer | 1×1012 – 1×1014 vg/mL |
| Purity | >95% (capsid proteins) |
| Transduction Efficiency | >70% in target T cells |
| Endotoxin Level | <5 EU/mL |
| Replication Competent Virus | Not detected |
"Creative Biolabs' AAV packaging service allowed us to efficiently generate CAR-T cells with high transduction efficiency. The technical support was excellent throughout the project." — P**S.
"We were impressed by the consistency of viral titers and the comprehensive quality reports provided. This platform significantly accelerated our CAR-T study timeline." — G**L.
"The team provided customized solutions for our CAR constructs and ensured successful in vitro and in vivo validation. Highly recommended for CAR-T development projects." — A**C.
Collaborating with Creative Biolabs means gaining a trusted scientific partner dedicated to advancing your CAR-T and AAV research goals. Our team combines deep expertise in viral vector engineering, cell therapy development, and analytical quality control to provide solutions that accelerate your innovation from concept to validation. Partner with Creative Biolabs — where precision meets innovation in viral vector and cell therapy development.
Which AAV serotypes can be used in your AAV packaging services?
Creative Biolabs offers packaging for a wide range of AAV serotypes, including but not limited to AAV1, AAV2, AAV5, AAV6, AAV8, and AAV9.
What tests are included in QC assays?
Our QC assays include tests for purity assessment using SDS-PAGE and HPLC, residual plasmid DNA analysis, residual host cell DNA and protein analysis, virus titer using qPCR and ELISA, sterility and endotoxin testing, among others. Each assay is designed to ensure the highest quality and compliance with regulatory standards.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION