Chimeric Antigen Receptor (CAR) T cell therapy has shown curative potential for B cell malignancies and is expected to treat other hematological malignancies and solid tumors. However, the general applicability of this treatment method is limited by the production costs associated with the expansion of the culture of the required number of CAR-T cells to achieve the therapeutic effect. In addition, studies have shown that systemic toxicity is caused by dose-related off-target transmission of these cytotoxic lymphocytes. Therefore, to optimize the applicability of CAR-T cell therapy, the key is to design strategies to improve the focus-specific colonization of this "living drug", thereby improving the efficacy and reducing production costs and the incidence of adverse events. It is well known that the tumor regression through intravascular administration of CAR-T cells depends on the ability of the cells to infiltrate the affected area. Therefore, improving the homing ability of CAR-T cells is essential for accurately targeting hematological malignancies.
To this end, Creative Biolabs provides fucosylation modification services on the surface of CAR-T cells to achieve enhanced homing ability. Research shows that fucosylated human CAR-T cells penetrate the tumor with a 10 times higher efficiency than non-fucosylated cells. Collectively, this novel strategy shows that the defect of CAR-T cell homing can be compensated by cell surface glycan engineering, and thus improve the colonization ability of CAR-T cells.
Glycosylation is an important post-translational modification that produces a diverse and abundant glycan pool (collectively called glycogen) on the cell surface. When focusing on immunity, glycans are essential in virtually all signal transduction and cell-cell interactions. More specifically, glycans have been shown to regulate key pathophysiological steps within T cell biology, such as T cell development, thymocyte selection, T cell activity, and signal transduction, and T cell differentiation and proliferation. They are essential for determining the interaction between human T cells and tumor cells.
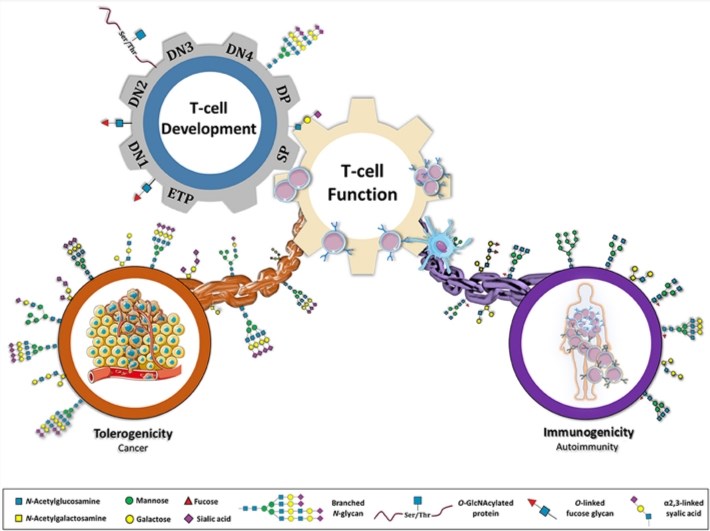 Fig.1 Glycans as a major connective chain that controls T cell response.1
Fig.1 Glycans as a major connective chain that controls T cell response.1
Although the role of glycosylation in T cell development and thymus selection remains to be fully understood, some important findings highlight the relevance of glycans in this process (Figure 2). In the field of protein biopharmaceuticals, such as anti-cancer antibodies, the potential of glycosylation engineering technology to enhance therapy has been extensively studied and utilized. So far, apart from removing or adding glycosylation sites, precise experimental adaptation of glycan structures on specific proteins in living cells is the least possible. Therefore, instead of focusing on specific glycoproteins, Creative Biolabs uses a systematic approach in which the glycan structure on the entire cell surface is disturbed. More specifically, we use CRISPR Cas9 gene editing technology to knock out genes related to glycosyl synthesis, such as Fut8, or other glycan-related genes of interest to customers.
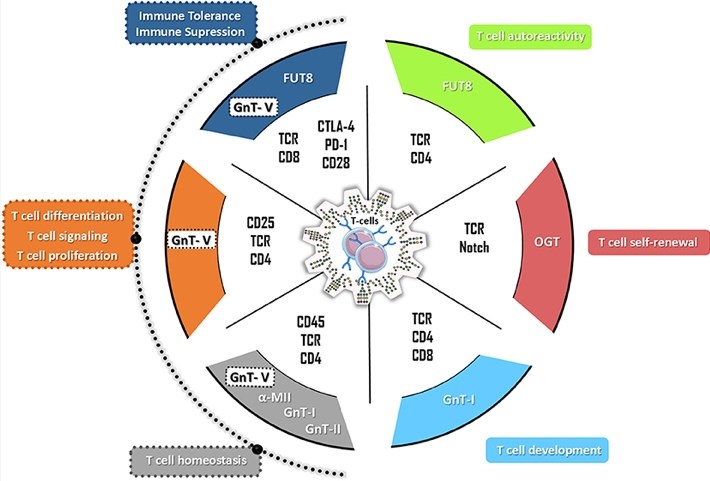 Fig.2 The hallmarks of glycans in T cell biology.1
Fig.2 The hallmarks of glycans in T cell biology.1
For more detailed information, please feel free to contact us or directly sent us an inquiry.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION