Engineered CAR-T cells offer highly specific targeting of disease-relevant cells or pathways, minimizing off-target effects.
Developing effective hemophilia treatments requires overcoming challenges in sustained therapeutic protein expression, precise gene delivery, and complex regulatory pathways. Creative Biolabs offers a robust CellRapeutics™ CAR-T development service to accelerate the development of targeted gene therapy for hemophilia diseases, providing highly specific and effective CAR-T cells for durable therapeutic outcomes through advanced CAR-T engineering, precise gene editing, and high-throughput screening platforms.
Hemophilia, a debilitating genetic bleeding disorder, typically requires lifelong clotting factor infusions. These treatments are costly, complex, and carry inhibitor risks. Gene therapy offers a transformative alternative by enabling endogenous clotting factor production, though durable expression and precise targeting remain challenges. Chimeric Antigen Receptor (CAR) T-cell therapy, known for oncology success, is now expanding to non-tumoral diseases like hemophilia. By engineering T cells to target specific pathways, we can achieve sustained, targeted therapeutic factor delivery or disease modulation. This innovative approach promises a long-term, potentially curative solution, overcoming conventional therapy limitations.
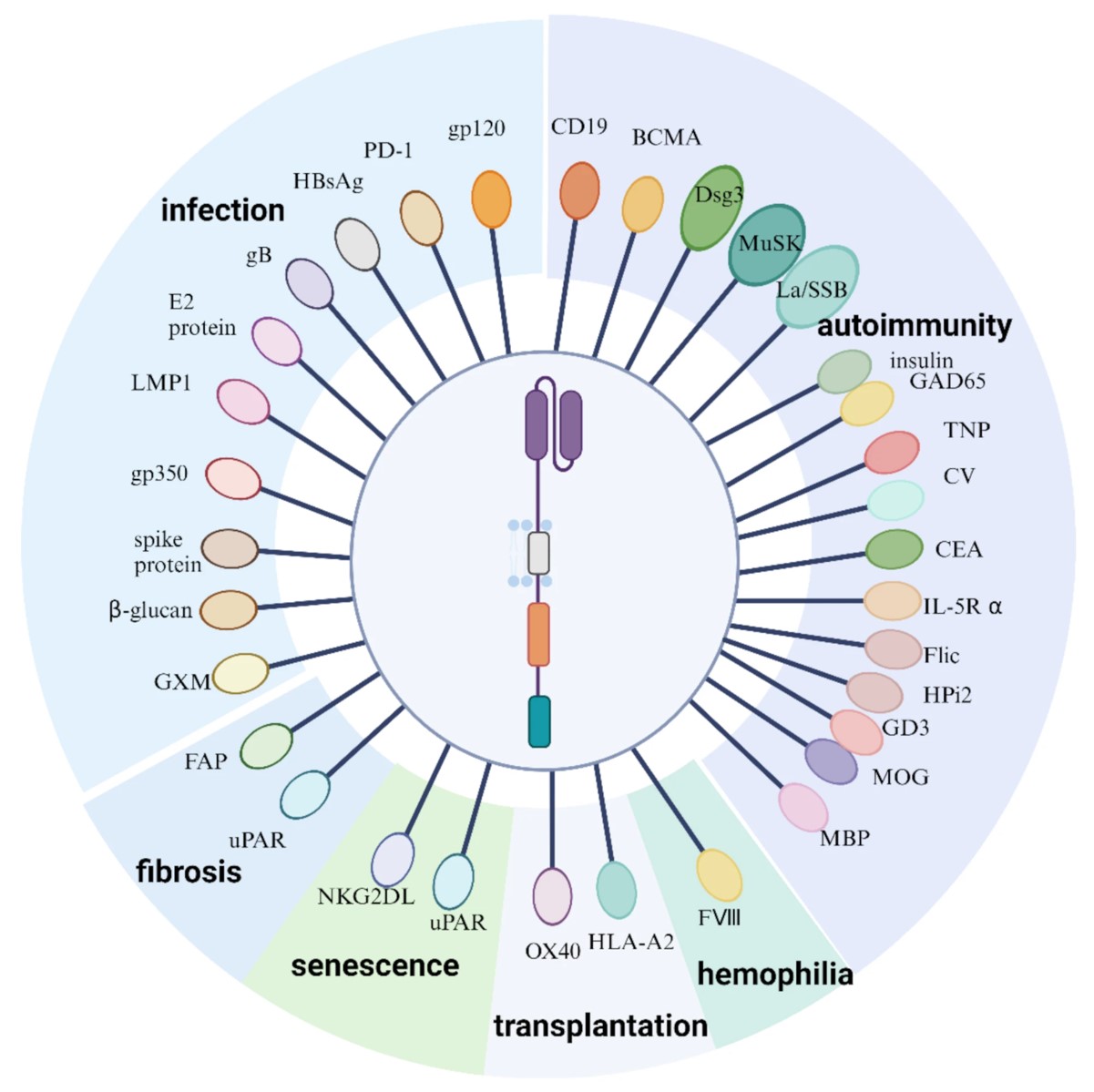 Fig.1 CAR immunotherapy's applications and various targets in non-oncology.1,3
Fig.1 CAR immunotherapy's applications and various targets in non-oncology.1,3
Creative Biolabs' CellRapeutics™ CAR-T development service provides a comprehensive, end-to-end solution for advancing your hemophilia gene therapy projects. We deliver highly characterized CAR-T cell constructs, robust preclinical data, and optimized protocols designed to accelerate your research and development pipeline. Our expertise ensures that your project is grounded in scientific rigor and tailored to your specific therapeutic goals.
Isolation of T cells from peripheral blood mononuclear cells (PBMCs) using advanced cell separation techniques. Subsequent activation of T cells using specific stimuli (e.g., anti-CD3/CD28 beads, artificial T cell stimulators) promotes proliferation and readiness for gene transduction.
Rational design of the CAR construct, including selection of optimal scFv (single-chain variable fragment) for target binding, hinge and transmembrane domains, and intracellular co-stimulatory domains (e.g., CD28, 4-1BB) coupled with the CD3ζ signaling domain. Gene synthesis and cloning of the designed CAR into a suitable lentiviral vector backbone.
Large-scale production of high-titer, replication-incompetent viral vectors encoding your specific CAR construct. This includes transiently transfecting packaging cell lines, followed by viral particle purification and concentration.
To stably incorporate the CAR gene, active T cells are transduced using the generated lentiviral vectors. Subsequent expansion of the CAR-T cell population under optimized culture conditions to achieve desired cell numbers and viability.
Comprehensive analysis of CAR-T cells including flow cytometry for CAR expression levels, T cell phenotype (e.g., memory vs. effector subsets), viability, and purity. CAR-T cell activity is assessed using functional tests such as cytokine release, cytotoxicity, and proliferation.
Evaluation of CAR-T cell efficacy in relevant in vitro models (e.g., co-culture assays with target cells expressing clotting factors or relevant pathways) and, if applicable, in vivo preclinical animal models of hemophilia to assess factor expression, coagulation parameters, and bleeding phenotypes.
Engineered CAR-T cells offer highly specific targeting of disease-relevant cells or pathways, minimizing off-target effects.
Potential for long-term, endogenous production of clotting factors, reducing the need for frequent exogenous infusions.
A single or limited number of treatments could potentially replace lifelong therapy, significantly improving patient quality of life.
Tailored CAR designs and development strategies to meet the unique requirements of your hemophilia research.
Rigorous quality control and functional testing ensure high-quality and effective CAR-T cell products.
In a recent example, autologous anti-CD19 CAR-T cell treatment resulted in full remission of an IST-resistant Acquired Hemophilia A (AHA) patient with persistent bleeding. This highlights CAR-T's promise as a safe and potentially cost-effective treatment for refractory AHA.
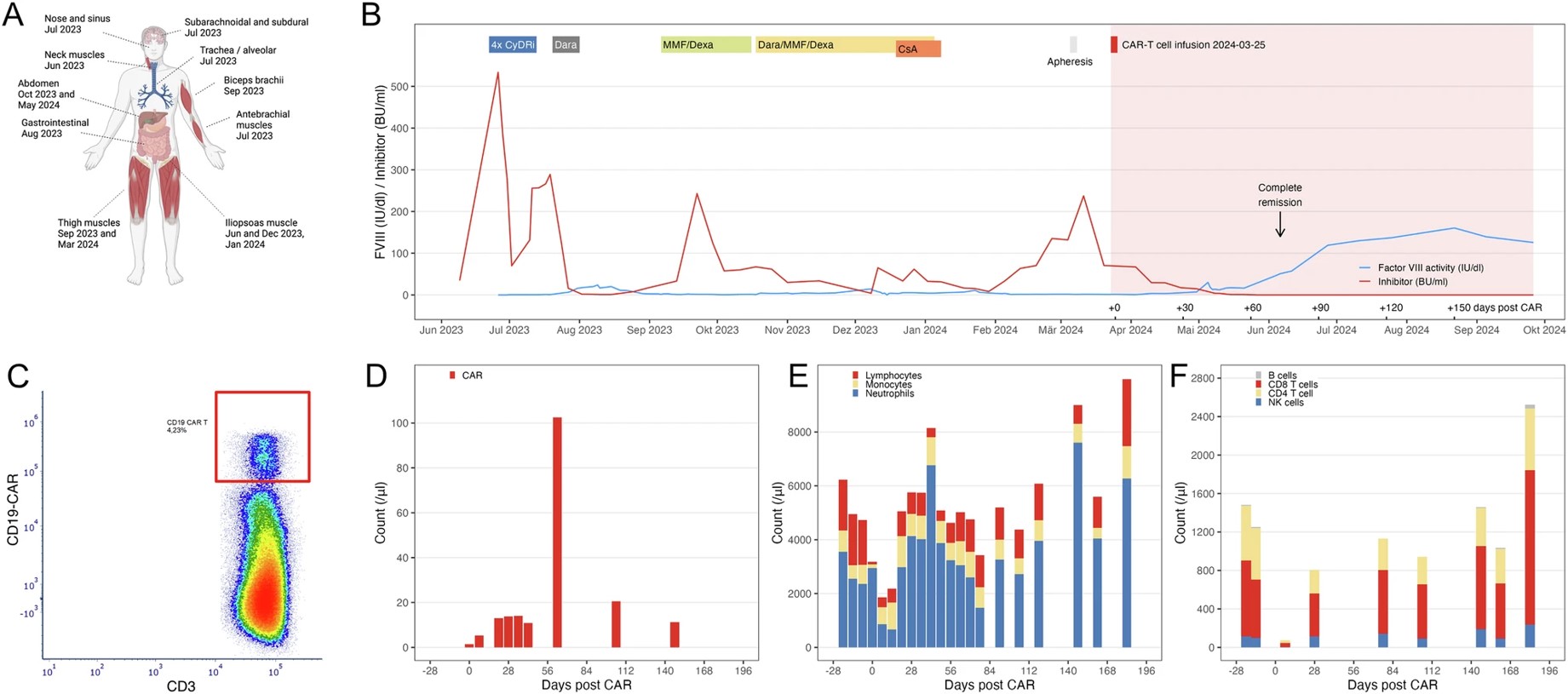 Fig.2 Anti-CD19 CAR-T cells' effect on acquired hemophilia.2,3
Fig.2 Anti-CD19 CAR-T cells' effect on acquired hemophilia.2,3
Q1: How does CAR-T therapy for hemophilia differ from traditional clotting factor replacement?
A1: Traditional treatments provide exogenous clotting factors, requiring frequent infusions. Our CAR-T therapy aims for a long-term, potentially curative solution by enabling the body to produce its own clotting factors, reducing treatment burden and improving patient quality of life. Contact us to learn more about this transformative approach.
Q2: How long does the CAR-T development process typically take, and what factors influence the timeline?
A2: The typical timeframe ranges from 12 to 20 weeks. This time frame can vary depending on the intricacy of the CAR construct design, the amount of T cell multiplication needed, and the degree of in vivo preclinical research. We deliver a complete project strategy and timeframe following an initial consultation.
In addition, Creative Biolabs offers a wide selection of supplementary services to boost your study:
Creative Biolabs is dedicated to accelerating the development of innovative therapies for hemophilia. Our CellRapeutics™ CAR-T development service for hemophilia diseases combines cutting-edge technology and exceptional knowledge to produce robust, dependable, and high-quality research solutions. For more details, please get in touch with us at your convenience.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION