To address the major issues of treating severe transplant rejection and persistent Graft-versus-Host Disease (GvHD), Creative Biolabs offers a CellRapeutics™ CAR-T development service for transplant rejection and GvHD. Conventional immunosuppressants are often burdened by systemic toxicity and limited efficacy. Our service helps you achieve targeted immune modulation and enhance transplant outcomes through advanced chimeric antigen receptor (CAR) T-cell engineering and precise cellular manufacturing.
Gene therapy is a rapidly developing field that presents previously unheard-of possibilities for the treatment of complicated illnesses. Despite advancements in transplantation, transplant rejection and GvHD remain significant hurdles, leading to patient morbidity and mortality. Current immunosuppressive regimens often lack specificity, resulting in broad immune suppression and associated side effects. Developing targeted CAR-T therapies represents a critical necessity to overcome these limitations, offering a precise, cell-based approach to modulate immune responses specifically against donor-recipient mismatches or alloreactive T cells, thereby improving patient safety and long-term graft survival.
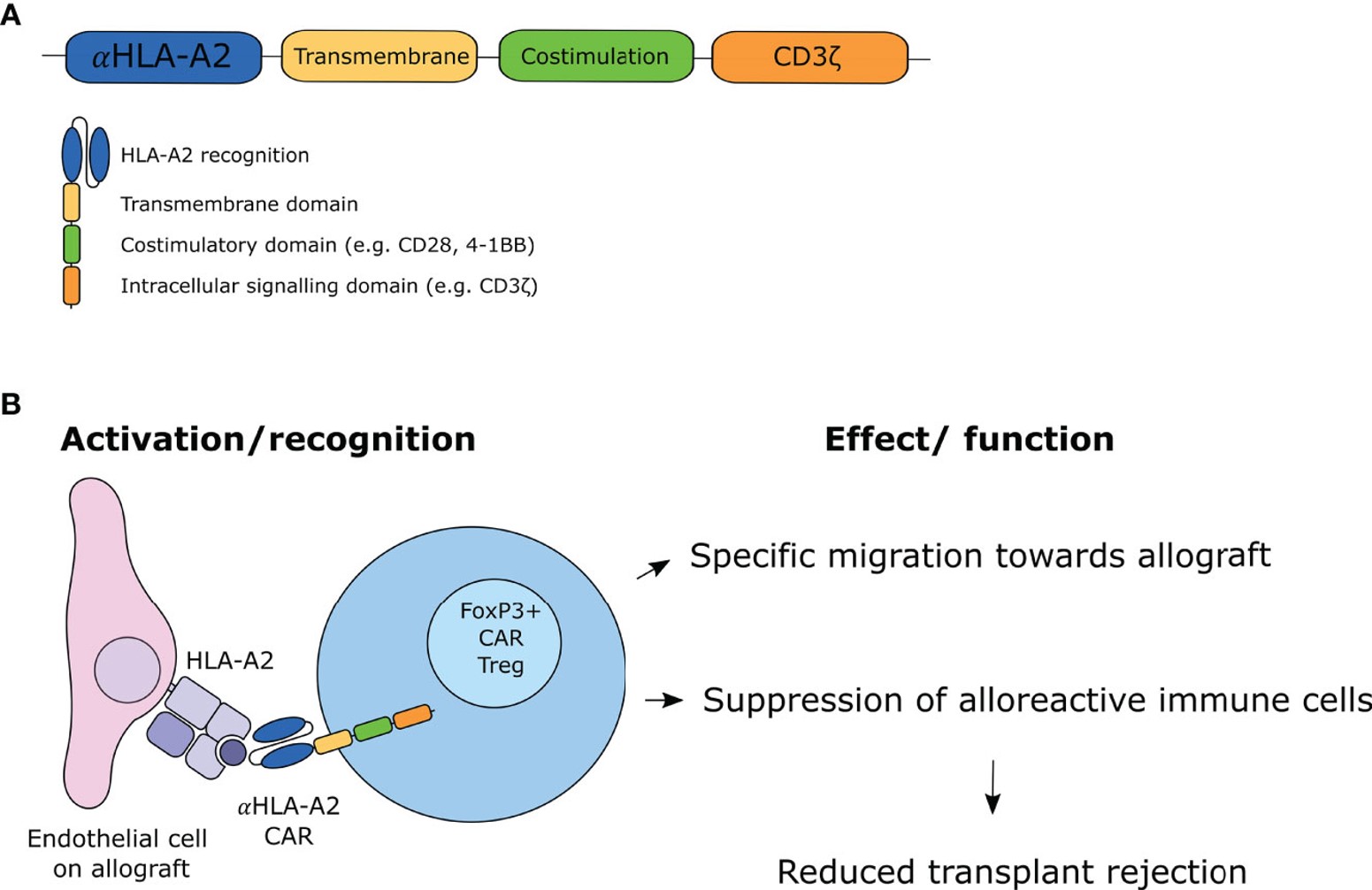 Fig.1 The design and mechanism of CAR Treg.1
Fig.1 The design and mechanism of CAR Treg.1
Creative Biolabs’ CellRapeutics™ CAR-T development service provides comprehensive solutions for addressing the complex challenges of transplant rejection and GvHD. We offer a tailored approach to engineer CAR-T cells designed to specifically target immune cells responsible for alloreactivity, providing a highly precise and effective therapeutic strategy. Our service delivers robust, high-quality CAR-T cell constructs and validated cell products, empowering your research and clinical development efforts.
We begin by collaborating with you to identify and validate optimal target antigens implicated in transplant rejection or GvHD. This involves comprehensive bioinformatics analysis and in vitro validation assays to ensure specificity and efficacy.
Our experts design and optimize the Chimeric Antigen Receptor (CAR) construct, including scFv selection, hinge, transmembrane, and co-stimulatory domains. Multiple designs are often explored to maximize CAR-T cell activation and persistence while minimizing off-target effects.
For effective gene transfer, clinical-grade, high-titer viral vectors (lentivirus or retrovirus) are produced. Strict quality control guarantees the safety, purity, and integrity of the vector.
Patient or donor T-cells are isolated, activated, and transduced with the optimized CAR construct. These CAR-T cells are then expanded ex vivo to achieve sufficient cell numbers and viability for downstream applications.
Extensive QC assays are performed, including CAR expression analysis, viability, purity, sterility, and in vitro functional assays (e.g., cytotoxicity assays against target cells, cytokine release, proliferation). In vivo proof-of-concept studies can also be conducted.
Q1: What is the typical turnaround time for a CellRapeutics™ CAR-T project?
A1: Project timelines vary depending on the complexity of your specific requirements, including target validation, CAR construct optimization, and the extent of preclinical validation desired. Generally, projects range from 12 to 24 weeks.
Q2: Can I integrate my own proprietary targets or constructs into your workflow?
A2:Our CellRapeutics™ CAR-T development service is designed to be highly flexible. We welcome collaboration on projects involving your proprietary targets, antibodies, or CAR constructs. Our team will work closely with you to seamlessly integrate your intellectual property into our robust development pipeline.
Creative Biolabs provides a range of supplemental services to help you with your cellular therapy research and development:
Creative Biolabs is a leader in the development of CAR-T cell therapy, with unparalleled experience and a successful track record. Our dedication to customer success and scientific excellence guarantees that your project is beneficial from state-of-the-art technology and exacting quality standards. If you would like further information, please get in touch with us whenever it is most convenient for you.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION