Lentiviruses are preferred viral vectors in gene therapy and CAR-T cell therapy due to their ability to integrate into the host genome, ensuring stable gene expression. Creative Biolabs, a leading biotechnology company, offers comprehensive CAR viral vector CDMO services. We provide cutting-edge lentivirus packaging services tailored for CAR-T cell therapy, ensuring high-quality and efficient viral vector production to meet the diverse needs of research and clinical applications.
Creative Biolabs boasts an advanced and flexible lentiviral packaging platform designed to meet the precise needs of our clients. Our platform is engineered to support various stages of CAR-T development, from research and preclinical studies to clinical trials and commercial-scale manufacturing. Our platform optimizes various parameters to enhance the efficiency and safety of lentivirus production. This includes advanced transfection protocols, scalable bioreactor systems, and automated purification techniques. This platform facilitates the production of high-titer, replication-deficient lentiviral vectors that are essential for the delivery of transgenes into CAR-T cells.
Our production system encompasses both adherent and suspension cell systems, offering versatility to accommodate various production scales and requirements. The adherent cell system is ideal for small to medium-scale production, providing ease of handling and consistency. For larger-scale production, our suspension cell system allows for scalable and high-density cultures, ensuring high yields of lentivirus. Additionally, we have established a fully developed cell bank system, which guarantees the reproducibility and reliability of our viral vector production processes. This cell bank system is meticulously managed to maintain cell health and productivity, further enhancing the reliability of our production processes.
To support seamless transitions from research to clinical applications, Creative Biolabs offers exhaustive process development services, encompassing upstream process development, downstream process development, and comprehensive quality control (QC) services.
This phase includes optimization of cell culture conditions, cell density, transfection plasmids and reagents, and virus harvest time. Our experts employ a combination of small-scale and large-scale production systems to refine and scale up lentivirus production processes efficiently.
Following production, our downstream process development focuses on purification and concentration of the viral vectors. Techniques such as tangential flow filtration (TFF), chromatography, and ultracentrifugation are employed to ensure high-purity vectors suitable for therapeutic applications.
A critical component of our service offering is the rigorous QC protocols to ensure the safety, potency, and quality of the viral vectors. This includes assays for measuring vector titers, potency, sterility, and the absence of RCL. Our QC team ensures that every batch meets the highest standards of quality and regulatory compliance.
Whether your needs are in research, clinical development, or commercial production, we are equipped to deliver high-quality, customized solutions to advance your projects to success. Contact us to learn more about our lentivirus packaging services.
Our comprehensive workflow covers every step of lentivirus manufacturing, ensuring consistent quality and efficiency from plasmid preparation to delivery.
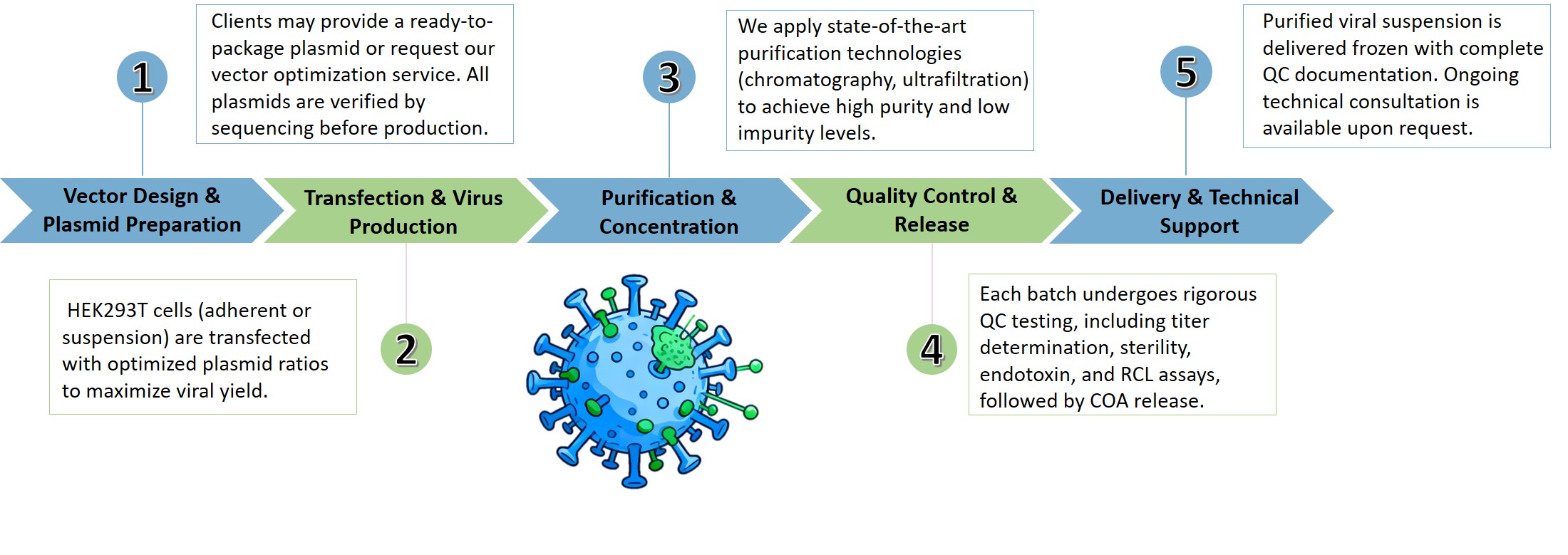
All lentiviral packaging at Creative Biolabs is performed in accordance with a robust quality management system that meets GMP-comparable standards. Our facilities and quality controls ensure the safety, purity, and potency of each viral batch.
| Quality Attribute | Test Method | Specification/Standard |
|---|---|---|
| Viral titer | Functional assay / qPCR | ≥ 1×10⁸ TU/mL |
| Sterility | USP <71> | No growth |
| Mycoplasma | PCR-based | Negative |
| Endotoxin | LAL assay | < 5 EU/mL |
| Residual host DNA/protein | qPCR / ELISA | ≤ 10 ng/mL |
| Replication-competent lentivirus | ELISA & qPCR | Not detected |
Our scalable lentivirus production platforms support a wide range of project needs, from early-stage research and development to late-stage clinical manufacturing. Both adherent and suspension HEK293 systems are available to ensure seamless scale-up and reproducibility.
| Production Type | Typical Volume | Application | Expected Titer |
|---|---|---|---|
| Research Scale | 0.1 L – 1 L | Functional validation, assay development | ≥ 1×108 TU/mL |
| Pilot Scale | 2 L – 20 L | Pre-clinical / IND enabling studies | ≥ 5×108 TU/mL |
| GMP Scale | 50 L – 200 L (suspension bioreactor) | Clinical & commercial use | ≥ 1×109 TU/mL |
What information should I provide before starting production?
Please provide the transfer plasmid sequence, the target gene, and the intended cell type. We can assist with vector optimization if needed.
Can you produce GMP-grade lentivirus suitable for clinical trials?
Yes. We offer full GMP-grade manufacturing under cleanrooms with complete batch documentation and RCL testing.
Do you support pseudotyping other than VSV-G?
Yes, pseudotypes such as BaEV, RD114, or amphotropic envelopes are available depending on your target cell preference.
"Creative Biolabs delivered high-titer lentivirus for our CAR-T cell research in record time, accelerating our critical timeline. The exceptional viral purity consistently exceeded our stringent QC standards, ensuring reliable performance in all our cell engineering experiments. This partnership was a key enabler for our project's success." — C**A.
"What truly distinguishes Creative Biolabs is their excellent communication and profound technical expertise. Their team provided invaluable guidance through every step, from initial plasmid design to final virus validation. This collaborative support was instrumental in de-risking our development process and achieving our goals efficiently." — S**S.
Partner with Creative Biolabs to accelerate your CAR-T and gene therapy development. With extensive expertise in lentiviral vector design, high-titer production, and GMP-compliant manufacturing, we provide flexible, reliable, and fully supported solutions from research to clinical stages. Let our experienced scientists help you achieve seamless project success. Contact us today to discuss your project needs and start your collaboration with Creative Biolabs.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION