Equipped with world-leading technologies and extensive experience in the field of antibody discovery and immunotherapy, Creative Biolabs now offers multiple premade CAR-T libraries. These robust libraries can be directly applied in functional CAR-T cells selection process to speed up our clients' projects for academic and clinical purposes.
1. Background
The classic strategy for a CAR vector construction is linking the gene of an antigen specific recombine scFv to a T cell activation gene in vitro. Then this synthesized artificial gene can be further studies. However, there are some shortcomings of this method. First, recombination with other genes may affect the expression of scFv fragments as well as their recognition of tumor antigens. Besides, the CAR expression level could be affected by some scFv fragments. Moreover, the activation of CAR-T cells is associated with the antigenic epitopes recognized by scFvs. Although some scFvs can recognize tumor antigens, they fail to activate T-cells efficiently. To overcome these problems, talented scientists from Creative Biolabs are working a way out by CAR-T library technology.
2. Introduction of CAR-T Library Technology
Our CAR-T library technology is a development of T cell display, which is a novel antibody selection strategy based on T cell activation. CAR genes with a large pool of antibody fragments (e.g. scFv, Fab, VHH, scFab) are constructed into vectors thus forming the CAR-T libraries. After transfection, T lymphocytes display CARs on their surface. This technology could ideally link the tumor specific antigen-antibody interaction with the effective activation of T cells. High-throughput screening methods are developed to obtain desired CAR-T clones with high specificity, high affinity, proper expression level, and effective activation of T cells. Then such clones can be used directly in preclinical animal assays and clinical experiments. Both of the two CARs currently approved in the market are targeting CD19. With the help of our superior CAR-T libraries, immunotherapy against multiple of other targets can be screened in a convenient way. Notably, except for CD19-CAR, we have generated several CAR-T libraries for solid tumors against different antigens, such as Her2, Her3, EGFR, FGFR1, VEGFR, etc.
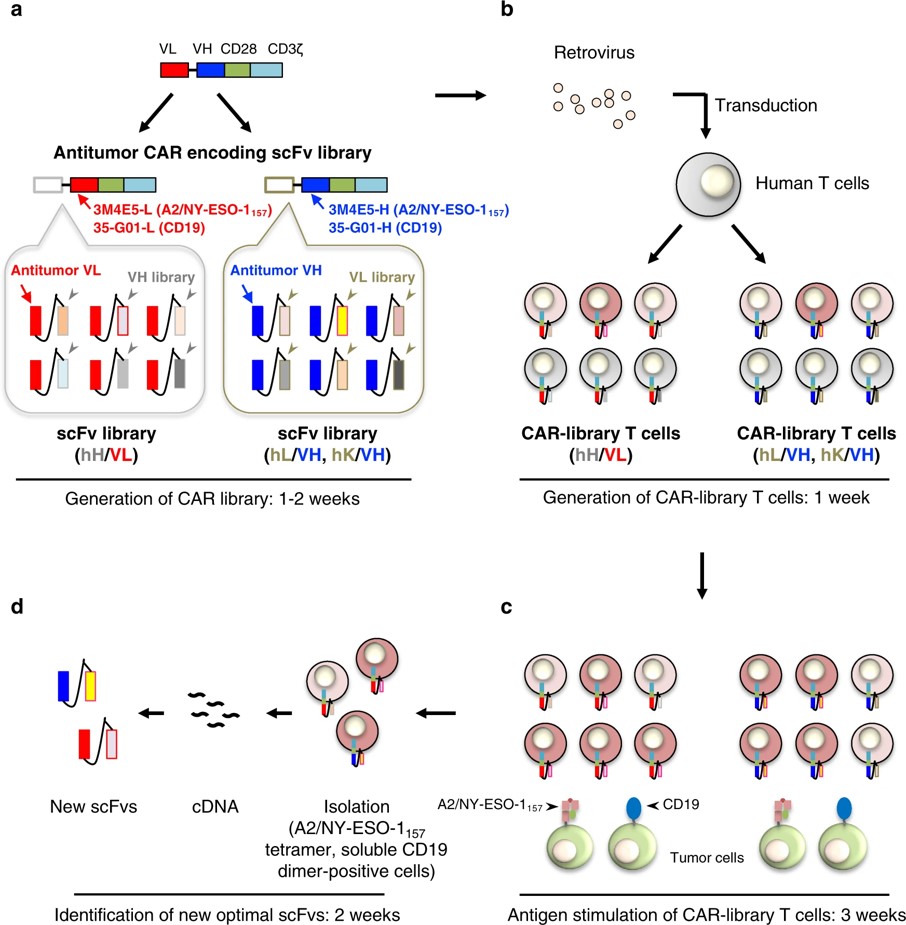
Fig.1 Identification of antitumor scFvs optimal for CAR-T cells using a T-cell-based scFv generation system.1
3. Highlight Features of Our Service
➢ Multiple antibody formats available
The antigen binding domains in our CAR-T libraries can be scFv, Fab, VHH, scFab, etc., providing flexible choices for our clients.
➢ Superior quality
Large library capacity is a key factor the success of display technology. The diversity of our CAR-T libraries is typically around 108, which is sufficient to generate highly affinitive binders from 10 pmol to nmol. Meanwhile, The selection is a positive selection system based on T cell activation, ensuring that isolated antibody fragments are functional within a CAR context.
➢ Time- and labor-saving
The stable and functional clones selected by our high-throughput screening can be used in clinic trials immediately, thus shortening the CAR-T cell immunotherapy development process.
➢ Ph.D. scientific team
Timely troubleshooting is promised to guarantee the highest success rate of each project.
Over years in the field of immunotheray, Creative Biolabs has served numerous customers all over the world. We are more than happy to share our technologies, platforms, and experience to facilitate your projects and push the progress towards clinical trials. Please feel free to contact us for more details and our team will get back to you as soon as possible.
Creative Biolabs has developed an innovative method based on the CRISPR/Cas9 library system to screen novel therapeutic targets in T cells that affect the infiltration of T cells into tumors. For more details, please visit: T Cell Homing Gene Identification with CRISPR/Cas9 Library
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION