CAR-T (Chimeric Antigen Receptor T-cell) therapy has revolutionized the treatment of various types of cancer, offering a promising avenue for patients with limited or no other treatment options. However, the success of CAR-T therapy is accompanied by the challenge of managing side effects, particularly Cytokine Release Syndrome (CRS). CRS is a systemic inflammatory response that can occur following CAR-T cell infusion and is a critical concern in the field of CAR-T therapy. Creative Biolabs, with our extensive experience and expertise, provides a specialized CAR-T Therapy-associated CRS assay service to address this crucial aspect of CAR-T treatment.
The goal of the management of CRS is to avoid harmful toxicities while maximizing the anti-tumor effect of cellular therapy. Location of care, supportive care, anti-IL-6 therapy, and glucocorticoids are four major approaches. The technical difficulties and cost inherent to real-time monitoring of serum cytokines have precluded the clinical application of this methodology to identify evolving CRS. At present, the use of C-reactive protein (CRP), which is made by hepatocytes in response to IL-6, becomes a laboratory marker of CRS onset and severity.
Creative Biolabs' CAR-T Therapy-associated CRS assay service is designed to assist researchers, clinicians, and pharmaceutical companies in assessing and managing CRS associated with CAR-T therapies. Our service comprises various components:
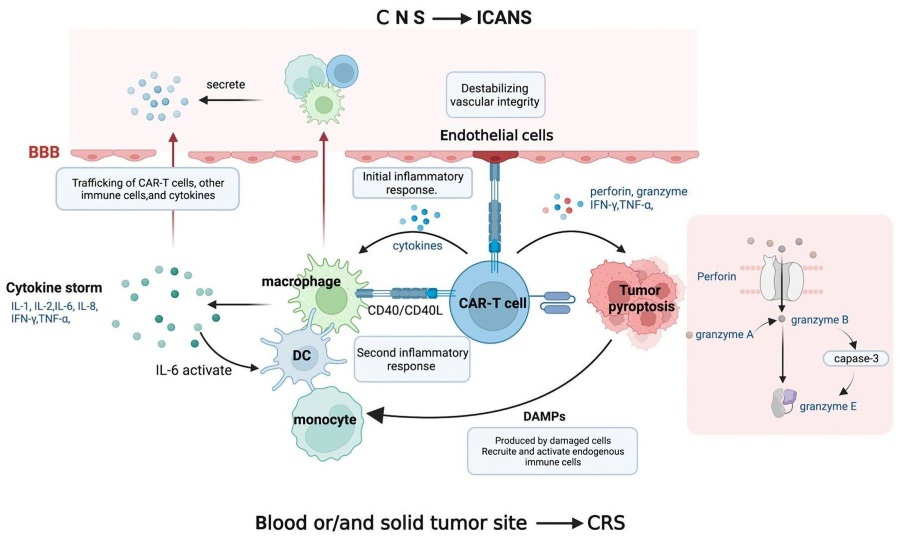 Fig.1 Potential pathophysiology of CAR T-cell mediated CRS.1
Fig.1 Potential pathophysiology of CAR T-cell mediated CRS.1
Creative Biolabs has multiple cytokine analysis platforms such as the multiplexed immunoassay platform, multi-analyte profiling platform, and cytokine profiling array. Each platform possesses its unique advantages. According to your project, we will select the appropriate technology and suitable assays combination to help you obtain higher-quality scientific research data. Besides, we also provide various cytokine profiling antibody array products for protein expression profiling of cytokines and related biomarkers.
Please contact us for more information about the different assays or services that are available to support your programs.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION