Creative Biolabs is a prominent provider of Contract Development and Manufacturing Organization (CDMO) services, focusing on the production of CAR viral vectors on a global scale. Our expertise encompasses the full spectrum of viral vector services, including the design, development, and manufacturing of high-quality retroviral and lentiviral vectors tailored for CAR-T therapy. Utilizing cutting-edge facilities and a rigorous commitment to Current Good Manufacturing Practices (CGMP), we guarantee that our viral vectors uphold the highest standards of safety, purity, and efficacy. Our team of skilled scientists and industry experts works closely with clients to identify their unique requirements, delivering tailored solutions that expedite the transition from research to practical applications. By leveraging our global network and cutting-edge technology, we empower organizations to advance their CAR-T therapies and contribute to the future of cancer treatment worldwide.
Adenoviral Vectors
- Derived from adenoviruses.
- Non-integrating; can carry large DNA inserts (~7.5 kb).
- Gene delivery for therapeutic purposes, vaccine development.
Lentiviral Vectors
- Derived from lentiviruses.
- Can integrate into the host genome, allowing stable expression.
- Gene therapy, especially in stem cell and cancer therapies.
Adeno-Associated Viral (AAV) Vectors
- Derived from adeno-associated viruses.
- Small size (~4.7 kb), less immunogenic, can integrate into the host genome.
- Gene therapy for inherited genetic disorders, ocular diseases, and more.
Retroviral Vectors
- Derived from retroviruses.
- Integrates into the host genome; limited to dividing cells.
- Gene therapy in hematopoietic stem cells.
Herpes Simplex Viral (HSV) Vectors
- Derived from herpes simplex virus.
- Can carry large fragments of DNA; can establish latency.
- Gene therapy for neurological disorders and some cancers.
At Creative Biolabs, we deliver end-to-end viral vector development and manufacturing solutions tailored for CAR-T and CAR-NK programs—from early design to GMP production. Our platform integrates vector design optimization, process scale-up, and rigorous quality control to help you accelerate clinical readiness while ensuring product safety and consistency.
What you can expect:
Our mission is to help you shorten development timelines, minimize manufacturing risk, and ensure the highest quality viral vectors for your CAR-based therapies.
Discover How We Can Help — Request a Consultation with Our Viral Vector Experts Today!
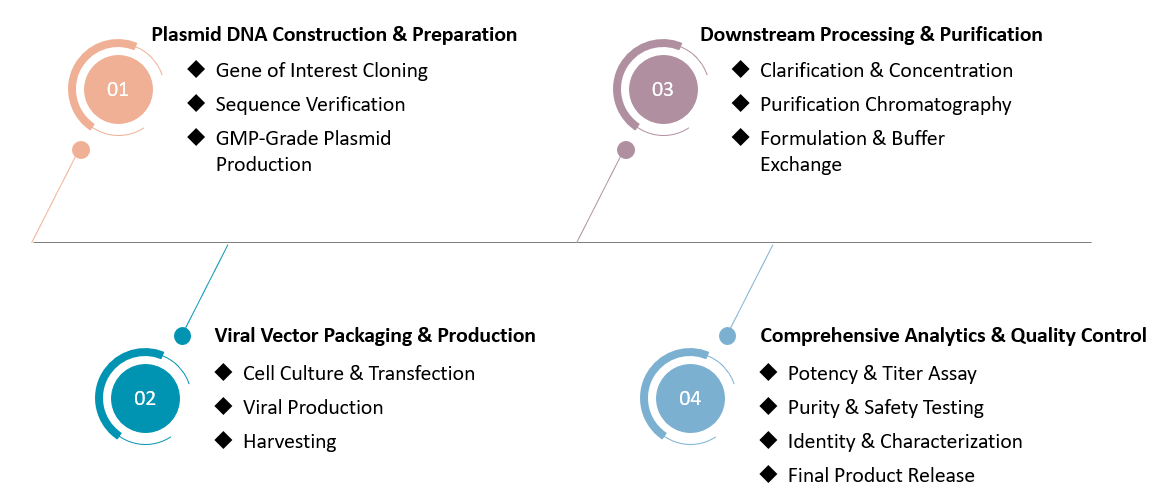
Our comprehensive CDMO platform ensures a streamlined and reliable pathway from gene of interest to high-quality viral vector, tailored for your CAR-T therapy development. To initiate vector manufacturing, clients typically provide:
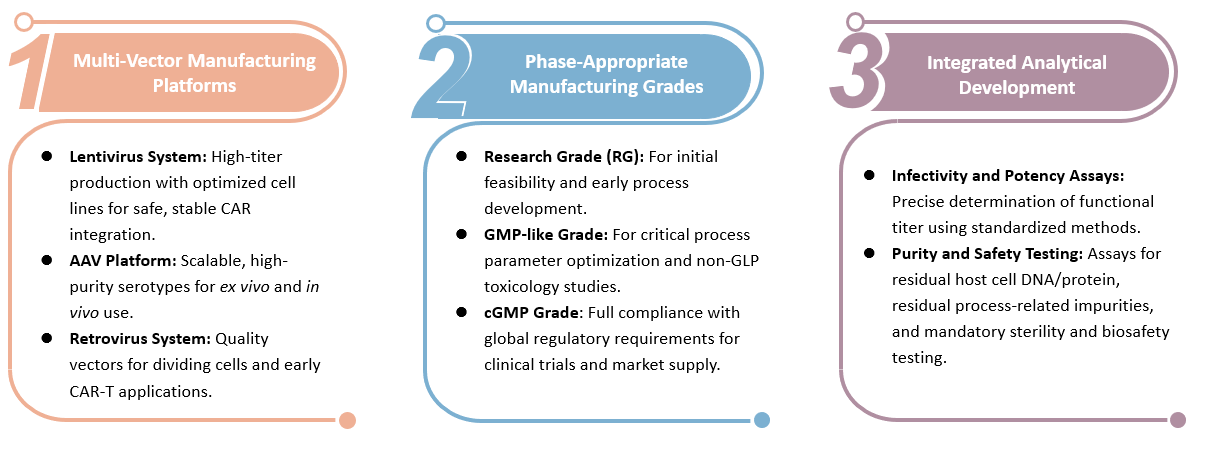
Research Grade (RG) Virus Manufacturing for CART
At Creative Biolabs, we specialize in providing advanced virus manufacturing services tailored for CAR-T therapy. Our state-of-the-art facilities and experienced team leverage cutting-edge technologies to produce high-quality viral vectors, ensuring optimal transduction efficiency and safety for CAR-T cell therapies.
Please click on the keywords above to get more information of interest!
GMP-like Virus Manufacturing for CART
At Creative Biolabs, we specialize in GMP-compliant Virus Manufacturing for CAR-T cell therapy, providing cutting-edge solutions that ensure the highest standards of quality, safety, and efficacy.
Please click on the keywords above to get more information of interest!
GMP Grade Virus Manufacturing for CART
Creative Biolabs provides tailored solutions that streamline the manufacturing process and support your research trials, enabling you to advance your CAR-T therapies with confidence.
Please click on the keywords above to get more information of interest!
Creative Biolabs offers comprehensive cGMP AAV production services tailored specifically for CAR-T therapies, ensuring compliance with the highest regulatory standards.
Please click on the keywords above to get more information of interest!
CGMP Lentivirus Production for CART
Creative Biolabs provides high-quality, clinical-grade lentiviral vectors tailored to meet the needs of cutting-edge gene therapies.
Please click on the keywords above to get more information of interest!
CGMP Retrovirus Production for CART
Creative Biolabs' CGMP retrovirus production services for CAR-T therapy provide high-quality retroviral vectors essential for the genetic modification of T cells.
Please click on the keywords above to get more information of interest!
Custom CGMP Virus Production for CART
Creative Biolabs provides tailored viral vectors, including both retrovirus and lentivirus, for the precise modification of T cells. Engineered, customized vectors that deliver specific genes into T cells, enabling them to recognize and eliminate cancer cells.
Please click on the keywords above to get more information of interest!
| Testing Category | Key QC Items | Routine Testing Methods |
|---|---|---|
| Sterility & Bioburden |
Sterility Test Bioburden |
Membrane Filtration Automated Microbial Enumeration |
| Mycoplasma | Mycoplasma Detection | Nucleic Acid Amplification Techniques |
| Viral Safety |
Endogenous & Adventitious Viruses Replication-Competent Virus |
In Vitro Assays (Co-culture) Animal In Vivo Assays Next-Generation Sequencing |
| Purity & Impurities |
Endotoxin Host Cell DNA Residue Host Cell Protein Residue |
Limulus Amebocyte Lysate Assay Quantitative PCR ELISA |
| Product Identity & Potency |
Identity Test Potency / Infectivity Assay Vector Titer |
Restriction Enzyme Digestion Cell-Based Infectivity Assay Quantitative PCR |

Ready to take your CAR viral vector production to the next level with our global CDMO services? Reach out to us today to explore how our extensive solutions can assist you in advancing your CAR-T therapy development from initial concept to successful commercialization. Our team of experts is committed to providing personalized assistance tailored to your unique project needs, ensuring high-quality viral vector production that meets global regulatory standards. Whether you have questions about our services, need advice on navigating the complexities of viral vector manufacturing, or are ready to initiate a partnership, we're here to help. Feel free to contact us by phone, email, or by completing the form below, and a member of our team will respond promptly to help you progress your CAR-T program.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION