Creative Biolabs is a leading biotechnology company in providing comprehensive CDMO services, particularly in the field of chimeric antigen receptor (CAR) viral vector packaging. By leveraging our extensive expertise and state-of-the-art technologies, we provide tailored solutions to meet the unique needs of each project, ensuring effective and efficient CAR virus production.
At Creative Biolabs, our CAR viral vector packaging services encompass two major platforms: adeno-associated virus (AAV) packaging and lentivirus packaging. Each platform offers distinct advantages tailored to different therapeutic applications.
AAV Packaging Services
AAV vectors are widely recognized for their safety and efficiency in gene therapy applications. Our AAV packaging services involve the production of high-titer AAV particles, designed for stable and efficient gene delivery. We employ advanced serotype selection techniques to meet the specific requirements of each project, ensuring optimal transduction efficiency and therapeutic outcomes.
Lentivirus Packaging Services
Lentiviral vectors are favored for their ability to integrate into the host genome, enabling long-term and stable gene expression. They can transduce a wide range of cell types, making them ideal for CAR-T cell therapies. Our lentivirus packaging services include the production of high-quality lentiviral particles with robust transduction capabilities.
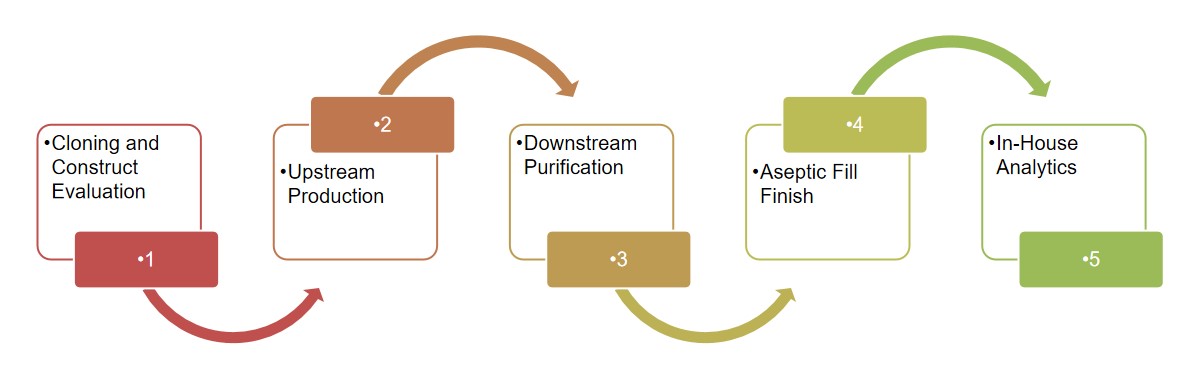
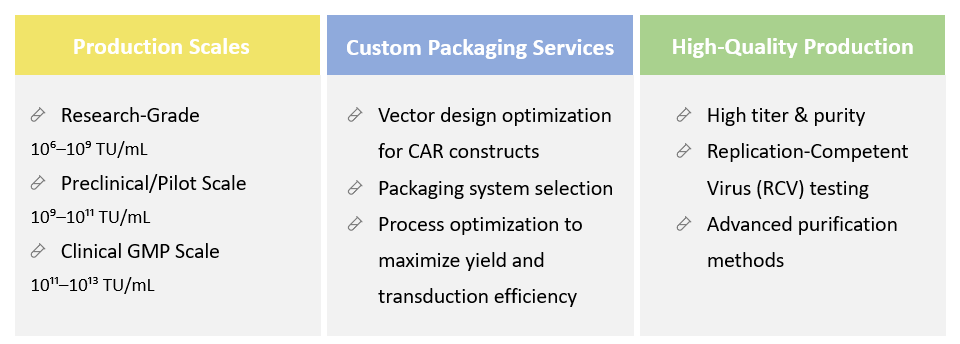
Creative Biolabs is proud to provide one-stop CDMO services that cover the entire spectrum of CAR viral vector packaging, from initial vector design and construction to large-scale manufacturing and quality control. The viral vectors we use adhere to GMP guidelines within state-of-the-art facilities. We provide customized process development services to optimize viral packaging. This includes designing scalable production processes, optimizing transfection protocols, and enhancing yield, titer, and purity. We conduct comprehensive QC tests to check for sterility, bioburden, endotoxin, etc. Our integrated services streamline the development process, minimizing the time and resources required to bring CAR-T therapies to market.
To support the rigorous demands of clinical and commercial production, Creative Biolabs operates a state-of-the-art GMP facility. Our GMP manufacturing services are designed to meet the highest standards of regulatory compliance and operational excellence.
Our facility is equipped with advanced bioprocessing equipment and controlled environments to ensure sterility and quality. We adhere to stringent GMP guidelines to produce viral particles that meet regulatory requirements and industry benchmarks for quality and safety.
Our GMP manufacturing services include large-scale production, downstream processing, fill-and-finish, and lot release testing. We have the capacity to support both small-scale clinical batches and large-scale commercial production, ensuring scalability and consistency. Our experienced team oversees the entire process, from raw material sourcing to final product release, maintaining the highest standards of quality throughout.
Can you provide vectors with specific serotypes or envelope proteins?
Yes, we support AAV serotypes (e.g., AAV2, AAV6, AAV9) and pseudotyped lentiviruses (e.g., VSV-G, RD114) to optimize transduction efficiency.
Are your viral vectors replication-competent virus (RCV) tested?
Absolutely. Every batch undergoes stringent RCV and adventitious agent testing before release.
Can you help with vector design optimization for CAR-T constructs?
Yes, our team provides vector sequence review, codon optimization, and packaging optimization for maximal yield and safety.
Do you offer support for regulatory documentation for clinical use?
Yes, GMP-grade vectors come with complete batch records, QC reports, and regulatory-compliant documentation.
"Creative Biolabs delivered high-quality lentiviral CAR vectors with exceptional titers. The team was responsive and provided detailed QC reports, making our preclinical studies seamless." D**L.
"The customization options were excellent. They helped us select the optimal packaging system and serotype for our CAR-T program. Highly recommend!" B**S.
"GMP-grade CAR viral vectors were delivered on time with comprehensive documentation, enabling a smooth IND submission. The support team is very knowledgeable." C**R.
With our expertise in AAV and lentivirus packaging and our commitment to delivering exceptional one-stop solutions, we empower our clients to advance their cell therapy programs with confidence. Partner with Creative Biolabs and experience the pinnacle of CAR viral vector packaging services designed to propel your therapeutic innovations forward. Contact us today to start your project.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION