Autoimmune diseases, characterized by immune system dysfunction leading to the attack of healthy tissues, pose significant challenges in medical treatment. Conventional therapies often target generalized immune suppression, presenting limitations in efficacy and potential side effects. The concept of utilizing CAR-modified Tregs offers a targeted approach to address autoreactive cells responsible for autoimmune responses. At Creative Biolabs, we are at the forefront of advancing the field with our innovative CellRapeutics™ CAR-modified Tregs Development Services.
CAR-modified Tregs are designed to express a Chimeric Antigen Receptor that enables them to recognize specific antigens associated with autoreactive cells. Unlike CAR T cell therapy for cancer, where effector T cells are deployed to target tumor cells, CAR Tregs exert their immunosuppressive effects on autoreactive cells within the local tissue microenvironment. By harnessing the specialized regulatory functions of Tregs, CAR-modified Tregs have the potential to restore immune balance and tolerance in autoimmune diseases.
Effector T cells (Teff) are known for their role in combating external threats, while Tregs act as immune regulators to prevent the body from attacking its tissues. Our approach involves engineering Tregs to express CARs that target self-antigens implicated in autoimmune responses. Through the bystander effect, CAR Tregs can suppress autoreactive Teff cells and promote immune tolerance at the tissue level.
The CellRapeutics™ CAR-modified T Regulatory Cells (Tregs) Development Services offer a comprehensive platform for the design, optimization, and production of CAR-modified Tregs tailored to specific autoimmune targets. At the same time, we also provide a series of relevant experiments to demonstrate and verify the relevant functions, and provide the optimal solution for the customer's project.
Our team of experts specializes in designing CAR structures optimized for autoreactive cells in a variety of autoimmune diseases. In addition to the customized development of special CARs for different targets, we also offer a variety of unique designs for customers to choose from in terms of internal CAR domains.
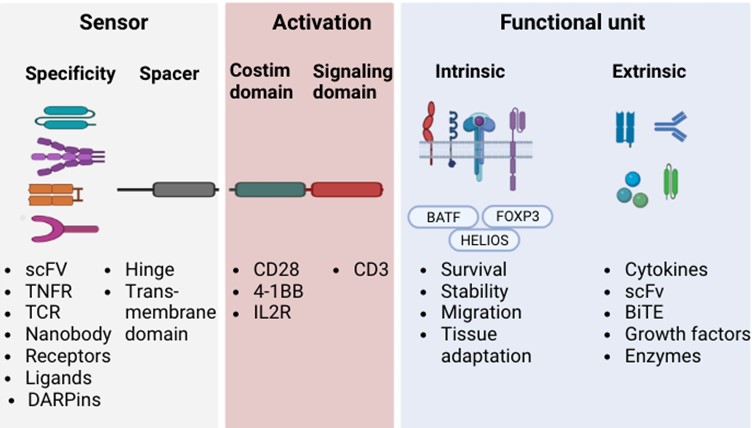 Fig.1 Diverse domain designs of chimeric antigen receptor (CAR) regulatory T (Treg) cells.1
Fig.1 Diverse domain designs of chimeric antigen receptor (CAR) regulatory T (Treg) cells.1
To evaluate the therapeutic potential and safety of engineered Tregs, we developed a series of in vitro and in vivo experimental platforms for validation. These validation tests are not limited to the following types:

Q1: What are the advantages and disadvantages of CAR-Treg strategies for treating autoimmune diseases compared to CAR-T strategies?
A1: Unlike traditional CAR T cell therapy, which eliminates pathogenic cells, CAR Tregs aim to promote tolerance and regulate the immune system by specifically targeting autoreactive cells. Advantages of CAR Tregs include their ability to exert antigen-specific immunosuppression, migrate to target organs, and potentially cause fewer side effects compared to polyclonal Tregs. CAR Tregs are also less dependent on IL-2 and can exhibit potent and specific immunosuppressive effects. However, there are limitations to consider, such as potential adverse reactions similar to those seen with anti-tumor CAR T cells, the need for specific antibodies to construct efficient CAR Tregs, and the risk of Treg exhaustion limiting their efficacy.
With the CellRapeutics™ CAR-modified T regulatory Cells (Tregs) development services, we hope to provide scientists with new ideas and more optimized solutions to open up new possibilities for treating autoimmune diseases. If you are interested in our services, please feel free to contact us.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION