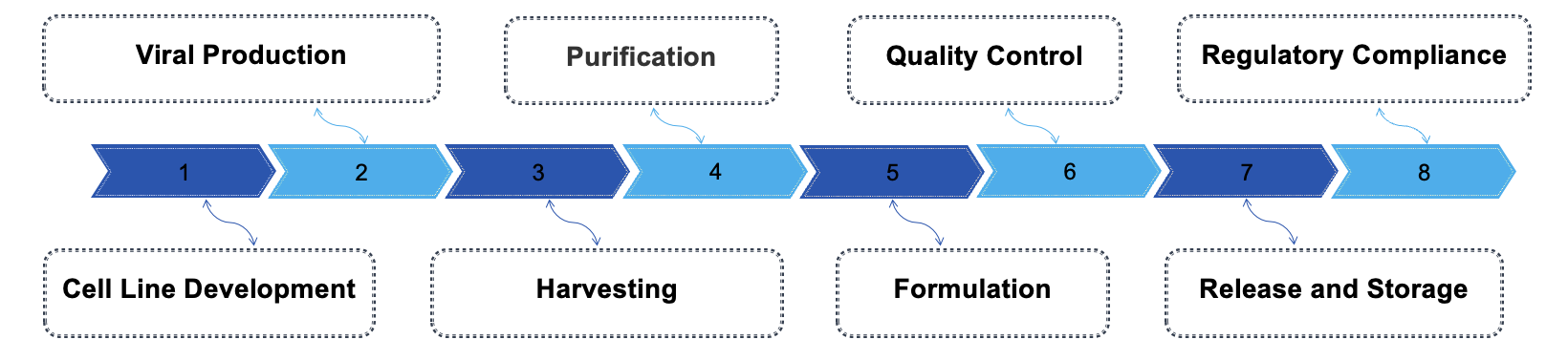
Creative Biolabs offers comprehensive cGMP AAV production services tailored specifically for CAR-T therapies, ensuring compliance with the highest regulatory standards. Our facility is equipped with advanced technologies for scalable production, from cell line development to purification and quality control.
CGMP AAV refers to "Current Good Manufacturing Practice" (CGMP) standards applied to the production of adeno-associated viruses (AAV) used in gene therapy and other biomedical applications.
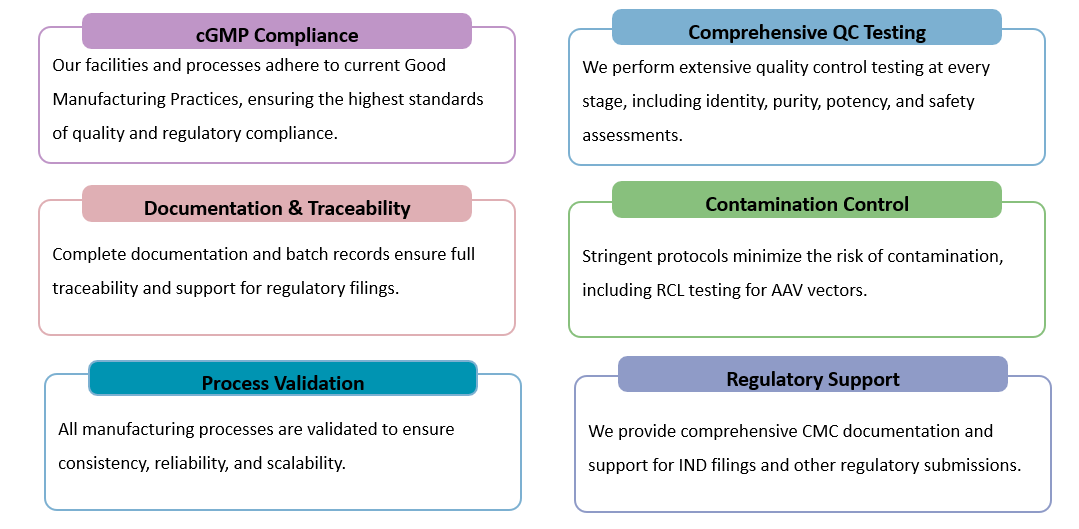
Importance of CGMP in AAV Production:
Our cGMP AAV production service for CAR-T therapies offers a robust and compliant solution for the manufacture of high-quality adeno-associated virus vectors. We utilize state-of-the-art technology and stringent quality control measures to ensure the safety and efficacy of the viral vectors. Our process encompasses everything from cell line development and transfection to purification and comprehensive testing, adhering to the highest regulatory standards. Emphasizing both scalability and customization, we collaborate closely with our clients to tailor our services to their unique requirements. Our support spans the entire process, guiding them from early-stage research through to clinical application, ensuring that their specific project goals are met at every step of development.
Experience the Creative Biolabs Advantage - Get a Quote Today
How does cGMP production impact CAR-T safety and efficacy?
cGMP ensures validated, documented processes that minimize contamination risks and guarantee consistent AAV titer/purity, leading to reliable T-cell transduction and predictable therapeutic outcomes.
Can you accommodate non-standard AAV serotypes for complex CAR constructs?
Yes. Our flexible service supports diverse AAV serotypes with customizable multi-plasmid transfection and pre-production optimization for maximum yield, regardless of construct complexity.
How do you ensure clinical-grade purity compared to research-grade AAV?
Our cGMP process uses optimized two-step chromatography (affinity and ion-exchange) to remove impurities and separate full/empty capsids, achieving superior full-to-empty ratios critical for clinical applications.
Using Creative Biolabs' cGMP CAR-T AAV Production Service in our research has significantly improved the consistency of our T-cell transduction rates. The ultra-high purity achieved via their chromatography process means we can use lower vector doses, reducing overall cost and improving the safety profile compared to our prior internal prep. Dr. Js Wn
We chose Creative Biolabs specifically for their cGMP documentation package. Their comprehensive batch records and QC data were exactly what our regulatory team needed, directly facilitating our IND submission. It was a massive time saver compared to generating the documents from scratch. Ra Sz
Our dedicated team is here to assist you with your cGMP AAV production requirements at every stage of the process. If you need more information, a personalized quote, or advice on how our services can support your project objectives, don't hesitate to contact us via phone or email. You can also complete the contact form on our website, and one of our specialists will get back to you swiftly. Let us collaborate with you to expedite your research and make a significant impact in the field of gene therapy!
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION