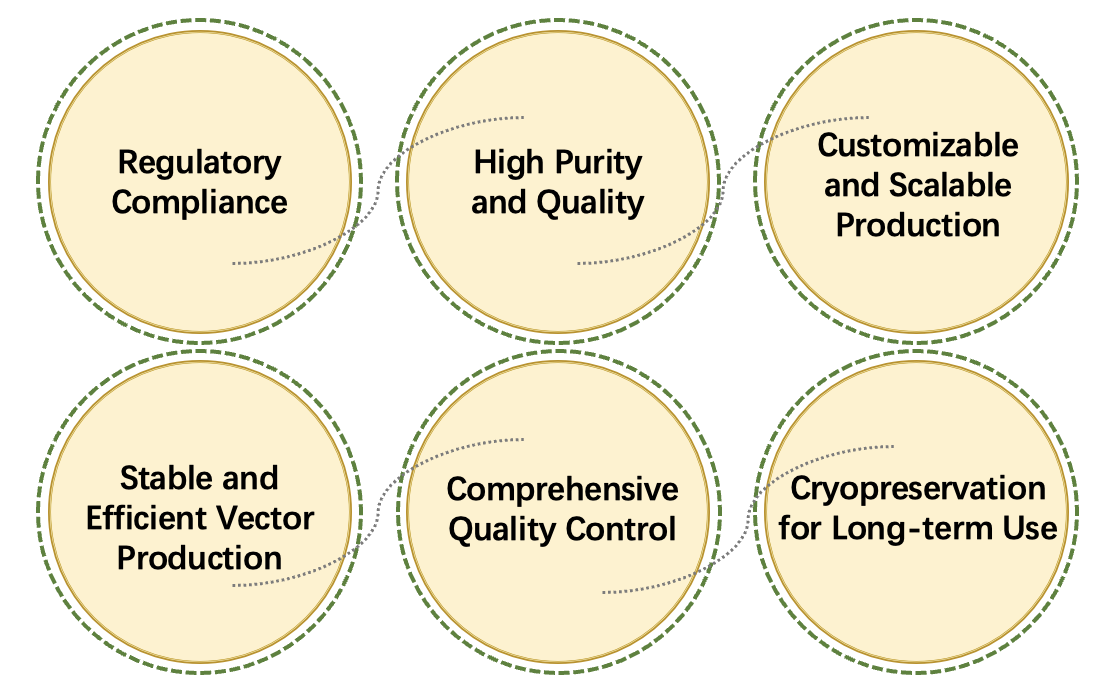
Creative Biolabs' cGMP retrovirus production services for CAR-T therapy provide high-quality retroviral vectors essential for the genetic modification of T cells. These retroviral vectors are designed to deliver the CAR gene into T cells, enabling them to specifically recognize and attack cancer cells. Adhering to strict CGMP, our service ensures the production of safe, consistent, and potent vectors suitable for clinical trials and commercial applications. From custom vector design to large-scale manufacturing and purification, CGMP Retrovirus Production services offer a complete solution to support the development of CAR-T therapies, ensuring regulatory compliance and high standards of quality control for successful therapeutic outcomes.
CGMP retrovirus is widely used in advanced therapeutic applications, particularly in CAR-T therapy, gene therapy, and immunotherapy. In CAR-T therapy, retroviral vectors deliver the CAR gene into T cells, enabling these engineered cells to target and destroy cancer cells. Additionally, cGMP retrovirus plays a key role in gene therapy by delivering corrective genes to treat genetic disorders, ensuring stable and long-term gene expression. Retroviral vectors are also utilized in the development of immunotherapies, stem cell modification, and vaccine production, making them essential for a range of gene and cell therapies. The cGMP-compliant production ensures that these vectors meet stringent safety, potency, and purity standards for clinical and commercial use.
Our CGMP Retrovirus Production service for CAR-T therapy is dedicated to providing high-quality retroviral vectors essential for the successful modification of T cells. Adhering to stringent CGMP, we ensure that our retroviral vectors meet the highest standards for safety, efficacy, and regulatory compliance. With customizable vector design, scalable production capabilities, and rigorous quality control processes, we deliver vectors that enable precise gene integration and enhanced therapeutic outcomes. Our expert team is committed to supporting your CAR-T therapy development, facilitating a seamless transition from research to clinical application.
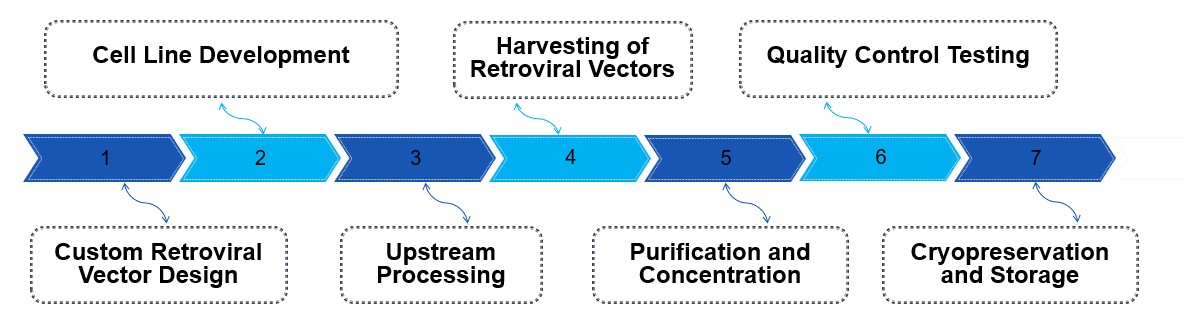
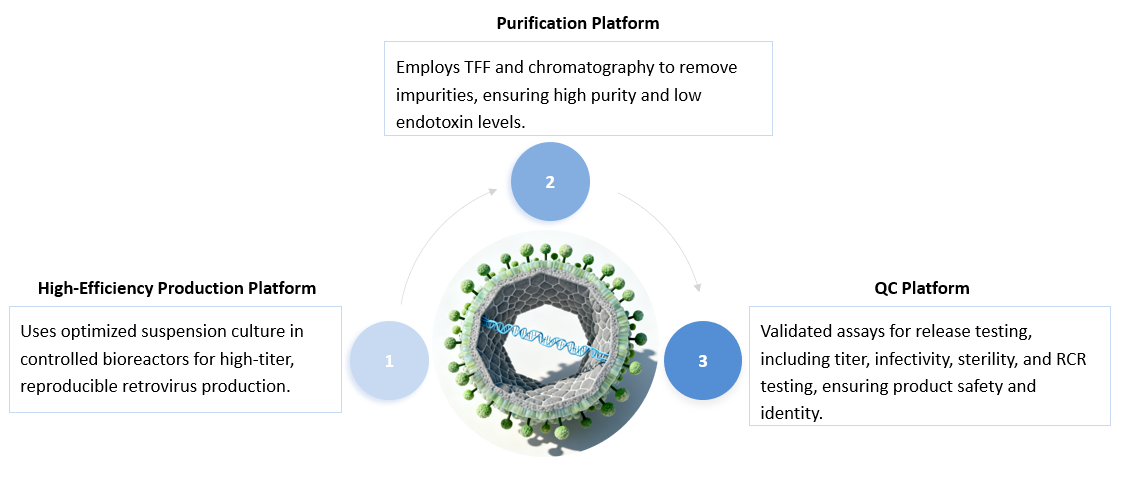
The process of CGMP retrovirus production starts with the custom design of the retroviral vector, incorporating the CAR gene and necessary regulatory elements. Stable producer cell lines are developed and cultured under strict cGMP conditions, followed by the transfection of these cells to produce retroviral particles. These particles are harvested, clarified, and undergo advanced purification techniques like filtration or ultracentrifugation to remove impurities. Comprehensive quality control testing is performed to ensure viral titer, purity, and safety, including checks for replication-competent retrovirus (RCR). The final product is cryopreserved for long-term storage, and all batches are fully documented to meet regulatory standards, ensuring readiness for clinical and commercial use in CAR-T therapies.
| Product Identity & Potency | Vector Titer | ≥ 1×109 TU/mL |
| Infectivity Ratio | Infectivity Ratio: < 50:1 (physical to functional particles) | |
| Transduction Efficiency | ≥ 70% (on primary T-cells) | |
| Safety & Purity | Sterility | Meets pharmacopeial standards |
| Mycoplasma | Not detected | |
| Endotoxin | < 0.1 EU / mL | |
| Replication-Competent Retrovirus | Not detected | |
| Host Cell Protein/DNA | < 50 ppm / < 5 ng / dose | |
| Process Consistency and Characterization | Empty Capsid Ratio | Controlled to specified limits |
| Vector Identity | Confirmed by restriction mapping | |
| pH & Osmolality | Within specified ranges |
What are the key differences between retrovirus and lentivirus vectors for CAR-T therapy?
The primary difference is that retrovirus vectors only infect dividing cells, while lentivirus vectors can infect both dividing and non-dividing cells. Retrovirus vectors have a simpler genome structure and are generally more cost-effective to produce. Lentivirus vectors offer a potentially safer integration profile with less risk of insertional mutagenesis. Both are effective for T cell engineering, with retrovirus vectors having a long history of successful use in approved CAR-T therapies.
Can you accommodate custom CAR constructs and specific vector designs?
Yes. Our flexible production platform can accommodate a wide range of CAR constructs and vector designs. We work closely with your team to understand your specific requirements and optimize our processes accordingly. Our scientists have extensive experience with various CAR formats, including second and third-generation designs, as well as different promoter and marker gene configurations.
How do you ensure the stability of retroviral vectors during storage and transportation?
We offer several formulation options optimized for retrovirus stability, including cryopreservation in appropriate cryoprotectants. Our vectors are typically stored at -80°C, and we provide validated stability data demonstrating retention of titer and functionality for at least 12 months under these conditions. For transportation, we use validated shipping containers with temperature monitoring to ensure product integrity throughout the supply chain.
"Using Creative Biolabs' cGMP CAR-T Retrovirus Production Service in our research has significantly improved the reproducibility of our CAR-T cell manufacturing process. We previously struggled with batch-to-batch variability in viral titer from other suppliers, which directly impacted transduction efficiency and experimental timelines. " Dr. Samanth
"The seamless transition from research-scale to clinical-grade material has been a key benefit. We had optimized our CAR construct using their research-grade retrovirus and were concerned about changes in performance when scaling up. However, the cGMP material demonstrated equivalent transduction efficiency and CAR expression, allowing our project to maintain its timeline. " Dr. Jenni
Ready to discuss your CAR-T retrovirus production needs? Contact our team of experts today.
Are you looking to advance your CAR-T therapy development with our cGMP Retrovirus Production services? Get in touch with us today to discuss your unique requirements and discover how our solutions can facilitate your projects. Our experienced team is ready to answer any questions you may have and offer personalized guidance to help you navigate the intricacies of retroviral vector production. Feel free to contact us by phone, email, or by filling out the contact form below, and we’ll get back to you quickly to support your therapeutic objectives.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION