Cytokine-induced killer (CIK) cells are a powerful immune cell population with potential in adoptive immunotherapy, but their efficacy and safety can be compromised by microbial contamination during culture or preparation. Understanding the importance of maintaining the highest standards of cell quality, Creative Biolabs provides comprehensive microbiological contamination testing to support the success of CIK cell-based therapies and experiments.
CIK cell therapy involves the expansion of immune cells in the laboratory, a process that requires rigorous control of the culture environment. During this process, the cells can become exposed to a variety of microbial contaminants, including bacteria, fungi, viruses, and mycoplasma. The presence of these contaminants can lead to compromised cell viability, altered functionality, or even failure of the therapy altogether. Moreover, microbial contamination can also pose a direct risk to patients when the contaminated CIK cells are infused into the patient's bloodstream, potentially causing infections or other serious complications. Therefore, ensuring the microbiological quality of CIK cell preparations is essential for both research and clinical applications. It ensures that the expanded CIK cells are free from harmful microorganisms, assuring that the therapy will not only be effective but also safe for patients.
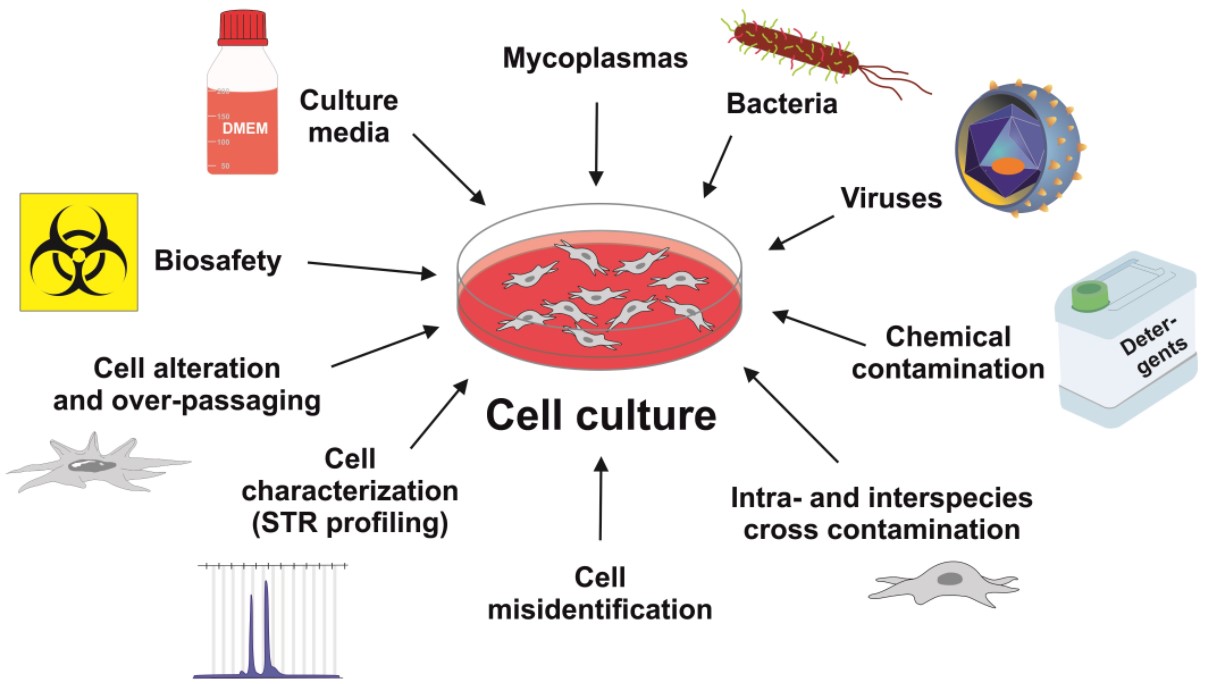 Fig.1 Cell regular testing for contamination and cell authentication testing.1
Fig.1 Cell regular testing for contamination and cell authentication testing.1
Creative Biolabs offers an advanced CIK microbiological contamination analysis service, designed to ensure the purity and integrity of CIK cells used in various research and therapeutic applications. Our team uses state-of-the-art diagnostic techniques, such as PCR and microbial culture-based methods, to accurately detect microbial presence in cell cultures. These methods are highly sensitive, ensuring that even low levels of contamination are identified. Our service includes a thorough screening for common microbial threats, including bacteria, fungi, yeast, and mycoplasma. Each of these contaminants can affect cell behavior differently, and their detection is crucial for ensuring the purity of the culture.
At Creative Biolabs, our CIK cell microbiological contamination analysis service provides an essential safeguard, ensuring that every batch of CIK cells is free from harmful microbial contamination. By combining cutting-edge technology with rigorous quality control, we are committed to helping our clients deliver safe and effective cellular therapies to patients worldwide. For more information about our microbiological contamination analysis services and how we assist with your CIK cell preparation, please contact Creative Biolabs today.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION