CRS is a potentially life-threatening systemic inflammatory response that can occur following CAR-T cell infusion, characterized by excessive release of pro-inflammatory cytokines. Creative Biolabs' CRS In Vitro Assay Services are designed to quantitatively evaluate the cytokine storm potential of immunotherapies prior to clinical application. These assays stand out due to its high sensitivity, multiplex cytokine profiling capability, and use of distinct immune cells, ensuring clinically relevant and predictive data. By utilizing this service, you will gain critical insights into the safety profile of their therapeutic products, optimize CAR-T cell design to mitigate CRS risks, and accelerate the development of safer and more effective cell-based immunotherapies.
Cytokines serve as pivotal mediators in CAR-T therapy, critically influencing T cell activation, persistence, and antitumor efficacy while also shaping the immunosuppressive tumor microenvironment. However, excessive or dysregulated cytokine activation can precipitate CRS, a systemic inflammatory response characterized by elevated levels of cytokines such as IL-6, IFN-γ, and GM-CSF, which may lead to life-threatening complications including vascular leakage, multi-organ dysfunction, and hemodynamic instability. Therefore, the implementation of robust CRS in vitro assays is essential for preclinically profiling cytokine storm risks, elucidating underlying immunopathological mechanisms, and guiding the development of targeted mitigation strategies to enhance both the efficacy and safety profile of cell-based immunotherapies.
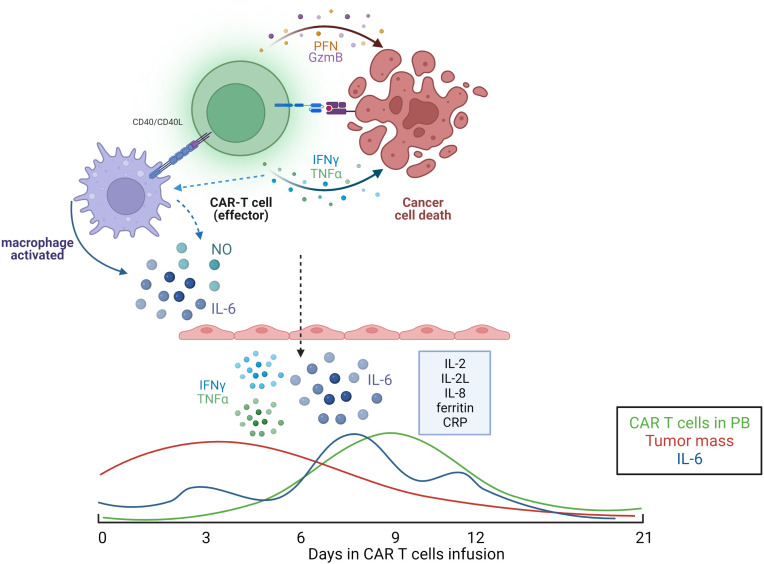 Fig.1 CAR-T cell therapy-induced cytokine release syndrome: mechanisms and kinetics.1
Fig.1 CAR-T cell therapy-induced cytokine release syndrome: mechanisms and kinetics.1
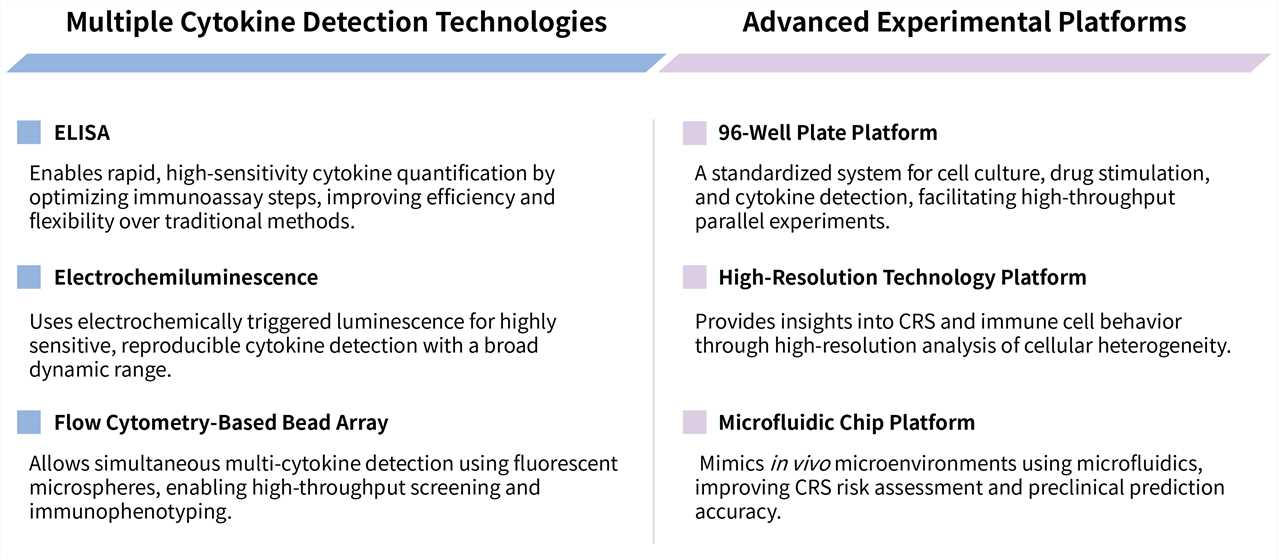
Creative Biolabs' CRS In Vitro Assay Services utilize an optimized co-culture system combined with high-throughput multiplex assay technology to deliver comprehensive profiling of the dynamic release kinetics of key cytokines. Our services provide you with robust and reliable data to gain critical insights into the CRS risk profile of your candidate therapy, thereby enabling you to refine product design and enhance its safety. Moreover, we offer in vitro cytokine release assay service and in vitro cytotoxicity analysis service, please feel free to contact us for more information.
We provide a comprehensive suite of advanced in vitro assay platforms to systematically evaluate and de-risk CRS, enabling precise profiling of immune cell activation, cytokine storm dynamics, and safety assessment for cell therapies.
Required starting materials:
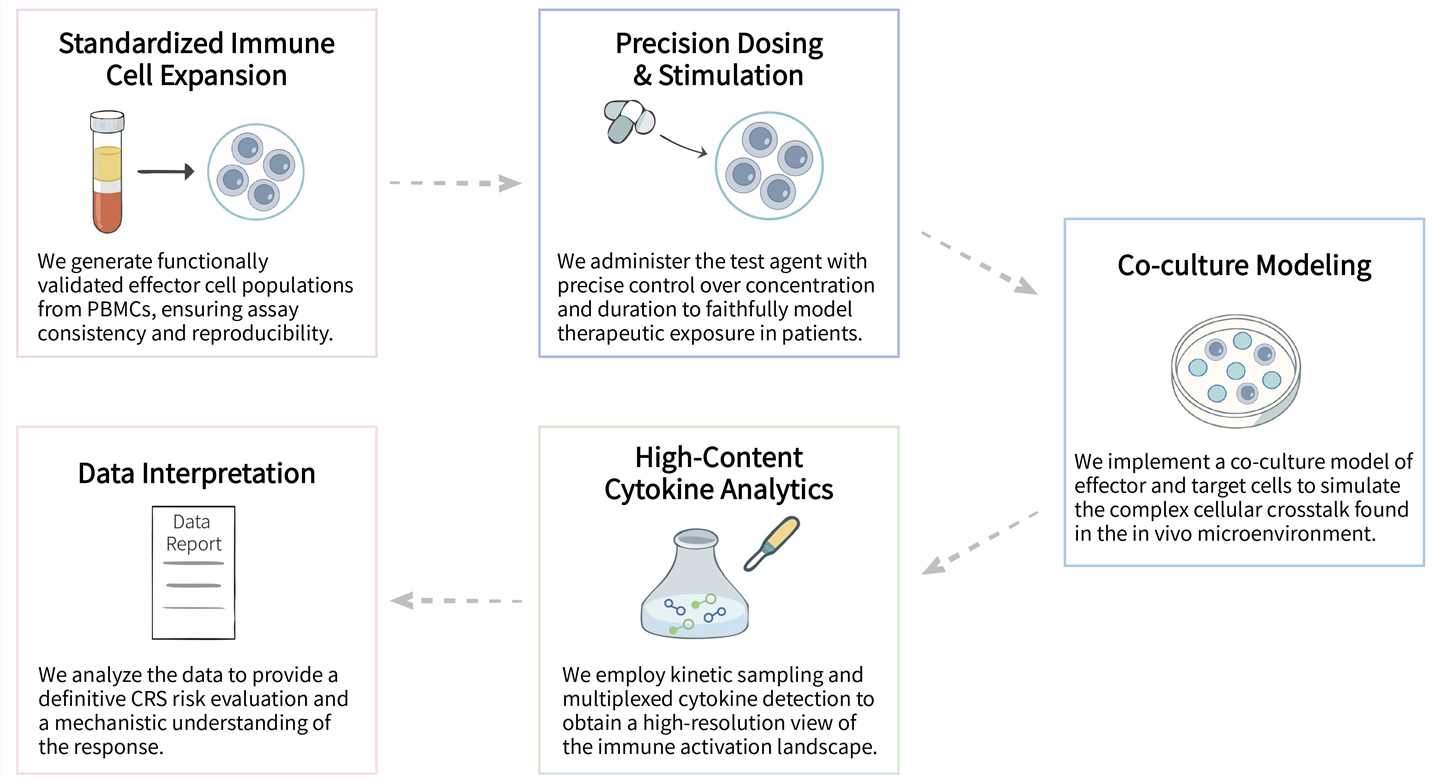
Final Deliverables: We provide a comprehensive data report including raw data files and quantitative parameters report.
Can your assay support the selection of a CRS mitigation strategy, such as co-administration of an IL-6 inhibitor?
Yes. Our mechanism-focused assays pinpoint the key pathways driving CRS, such as JAK1 or IL-6R, enabling you to evaluate your lead candidate alongside reference inhibitors within the CRS model. This allows determination of inhibitor IC50 in your specific therapeutic context, informing rational dose selection for combination therapy in early-phase trials.
What are the primary factors affecting the overall cost and timeline of your CRS In Vitro Assay service?
Cost and timeline are primarily determined by three variables: assay complexity, experimental throughput (number of agents and concentrations tested), and the scope of the readout panel. A detailed project proposal with pricing and schedule is provided after a complimentary consultation.
In the field of immunotoxicity screening, we stand apart through our superior predictive power, dedicated to providing data packages of exceptional reliability and practical utility. We have moved beyond the traditional paradigm of cytokine detection by adopting high-resolution technologies and constructing models with direct translational relevance, thereby guaranteeing both precision and actionable insights in research outcomes.
"Using Creative Biolabs' CRS In Vitro Assay in our research has significantly improved our ability to resolve T-cell vs. NK-cell cytokine contribution via Intracellular Cytokine Staining. This level of mechanistic detail is impossible with standard ELISA and directly informed our lead selection."— Dr. K***ly N.
"We transitioned our CAR-T safety screening entirely to Creative Biolabs' autologous monocyte co-culture model after our internal PBMC assay failed to predict severe monocyte activation. Their model successfully isolated the IL-6 driven CRS signal, drastically de-risking our trials."— Ms. R***h S.
"Creative Biolabs provided a comprehensive data package that integrated quantitative IC50 values for key chemokines (CCL8, CXCL10). Their data quality is non-negotiable."— Prof. J***n M.
Leveraging deep-seated scientific knowledge and cutting-edge technological platforms, we deliver bespoke solutions tailored to each project's unique requirements, ensuring objectives are not only met but surpassed. To explore how our expertise can advance your goals, we invite you to connect with our team.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION