Creative Biolabs provides tailored viral vectors, including both retrovirus and lentivirus, for the precise modification of T cells. Engineered, customized vectors that deliver specific genes into T cells, enabling them to recognize and eliminate cancer cells. The production process follows strict current Good Manufacturing Practices (cGMP), ensuring the highest levels of safety, quality, and regulatory compliance. With scalable manufacturing, robust purification methods, and comprehensive quality control testing, custom cGMP virus production supports the unique needs of CAR-T therapy development, from early research through clinical trials and commercialization. This service ensures that the viral vectors produced are safe, potent, and ready for therapeutic use, accelerating the advancement of personalized cancer treatments.
Custom CGMP virus production for CAR-T therapy includes various types of viral vectors tailored for gene delivery in T cells. The primary types include:
Retroviral vectors are widely used to integrate the CAR gene into T cells. Retroviruses are particularly effective in gene therapy applications due to their ability to stably integrate genetic material into the host genome, ensuring long-term gene expression. cGMP retrovirus production focuses on producing high-titer, purified vectors that meet clinical-grade standards for CAR-T therapies.
You can click here to get more information!
Lentiviral vectors are another key option for CAR-T therapy, especially for their ability to transduce both dividing and non-dividing cells. Lentiviruses offer stable, long-term expression of the CAR gene and are favored for complex genetic modifications in T cells. cGMP lentivirus production ensures high-quality, safe vectors that are suitable for both research and clinical applications.
You can click here to get more information!
Adenovirus Production
While less common in CAR-T therapy, adenoviral vectors can be used for transient expression of the CAR gene. These vectors are non-integrating, meaning they do not modify the host genome permanently. cGMP adenovirus production focuses on providing high-quality vectors with controlled and transient gene expression, suitable for certain CAR-T applications or vaccine development.
AAV (Adeno-Associated Virus) Production
Though not typically used in CAR-T, AAV vectors are employed in gene therapy and can potentially play a role in delivering genetic material to modify T cells or in preconditioning patients' immune systems. cGMP AAV production services ensure high-purity and safe vectors for clinical use.
You can click here to get more information!
The transition from a promising CAR construct to a clinical-grade cellular therapeutic demands an assured supply of vectors that meet the highest standards of safety and potency. Creative Biolabs specializes in de-risking this critical step by providing custom-engineered, cGMP-compliant lentiviral and retroviral vectors, tailored precisely for your T-cell modification requirements.
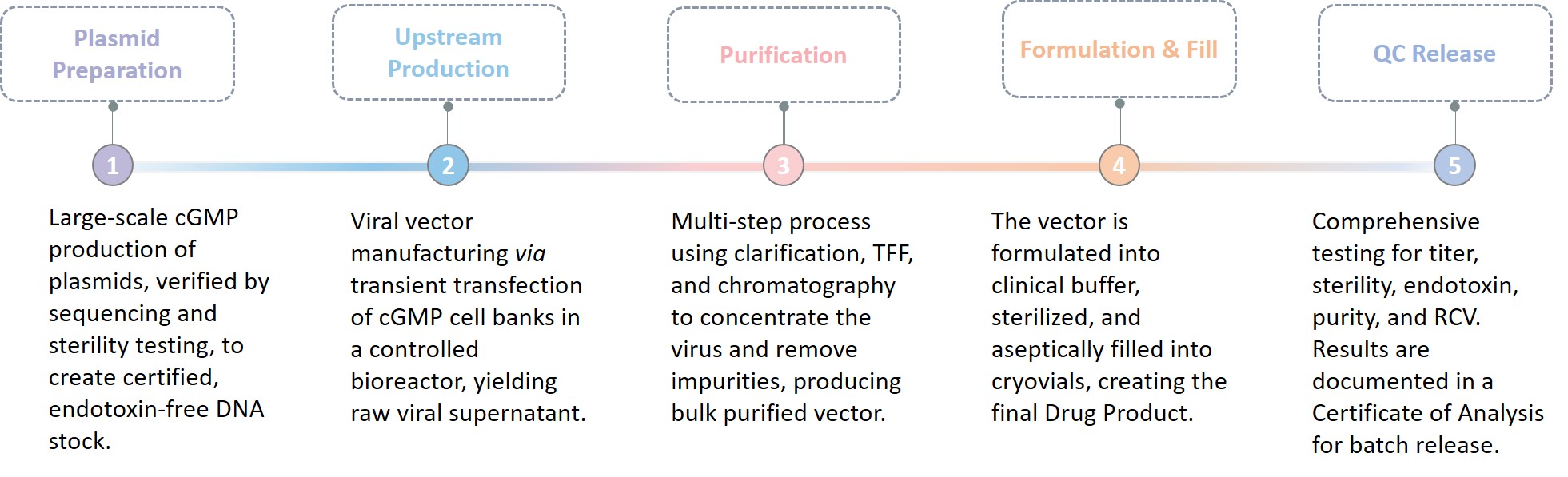
The typical manufacturing process is a phased, highly controlled sequence ensuring quality and consistency from start to finish.
Final deliverables include the cGMP viral vector, its quality control Certificate of Analysis (CoA), and a comprehensive Batch Record Report.
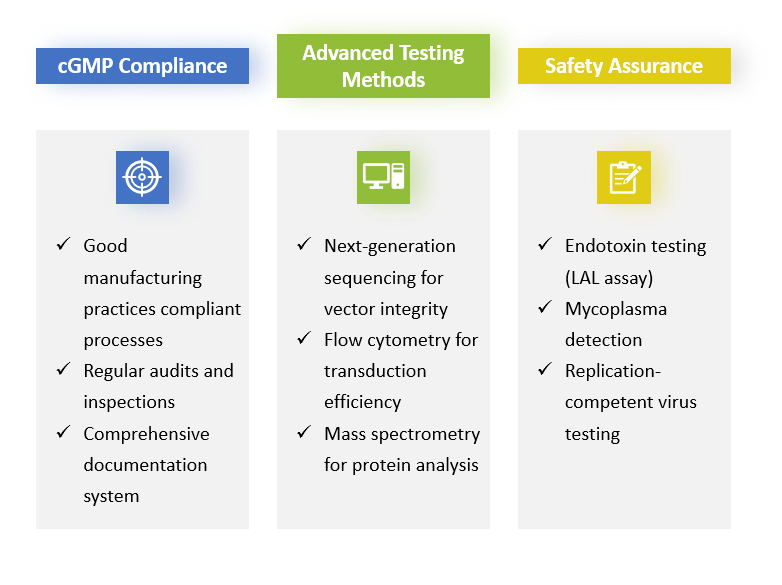
Quality Control and Testing
In-process and final product testing must comply with regulatory guidelines. Key tests include:
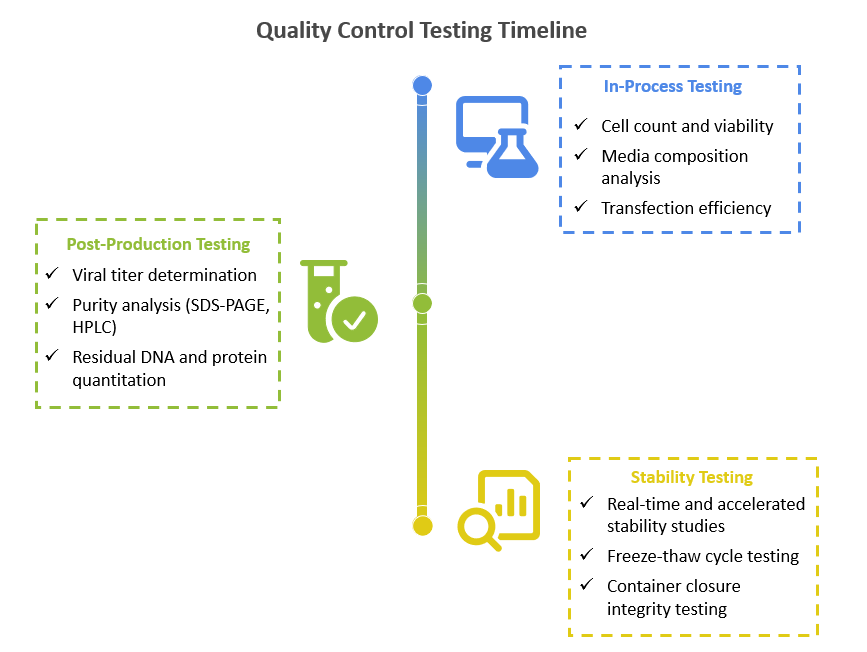
Quality Control Testing Timeline
Experience the Creative Biolabs Advantage - Get a Quote Today
What is the risk associated with Replication-Competent Virus (RCV), and how does Creative Biolabs mitigate this in your production process?
RCV is a critical safety hazard. We mitigate it using RCV-free cell lines, self-inactivating vector designs, and a sensitive, validated RCV assay on every batch.
Can your cGMP process be adapted for unique or fourth-generation CAR constructs, such as those with co-stimulatory domains or safety switches?
Yes. Our platform is highly flexible. We customize the packaging strategy to ensure stable, high-level expression of complex payloads like co-stimulatory domains or safety switches.
My allogeneic CAR-T project requires enormous vector quantities. Can your facilities handle the required scalability for commercial production?
Yes. Our scalable cGMP facilities and closed-system processes can handle lot sizes from clinical to commercial, ensuring consistent quality and yield for large-volume projects.
Using Creative Biolabs' Custom cGMP CAR-T Virus Production Service in our research has significantly improved our ability to transition smoothly from pre-clinical to IND-enabling studies. Their RCV testing is the most rigorous we've encountered, giving us complete confidence in patient safety. Dr. Al*on J.
The functional titer consistency from lot to lot has been a game-changer. We've seen a measurable improvement in T-cell transduction efficiency compared to previous manufacturers, allowing us to lower the vector-to-cell ratio needed for manufacturing. Prof. K*n L.
Contact Our Team for More Information and to Discuss Your Project.
Ready to explore how our Custom cGMP Virus Production services can accelerate your CAR-T therapy development? Contact Creative Biolabs today to discuss your specific project needs and learn how our tailored solutions can support your specific immunotherapy goals. Our experienced team is here to provide personalized guidance, answer any questions, and help you navigate the complexities of viral vector production. Reach out via phone, email, or by completing the contact form below, and we'll respond promptly to assist you in bringing your therapy to the next stage.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION