Are you currently facing complex challenges in developing safe and effective cell therapies, such as the unpredictable and potentially life-threatening risks of cytokine release syndrome (CRS) and neurotoxicity? Creative Biolabs' CellRapeutics™ Cytokine Release Test Service offers a comprehensive solution to identify and characterize the cytokine profile of your therapeutic candidates, helping you mitigate these critical safety concerns and accelerate your biopharmaceutical development pipeline.
The advent of CAR-T cell therapy has redefined the oncological treatment landscape, yet its clinical dividend is frequently curtailed by dose-limiting toxicities, most prominently CRS and immune-effector-cell–associated neurotoxicity syndrome (ICANS). These adverse events originate from the explosive, cascade-like liberation of pro-inflammatory cytokines such as IL-6, IFN-γ, triggered when CAR T cells engage and lyse their target cells, culminating in a systemic inflammatory storm. Owing to the unpredictable kinetics and potentially fatal trajectory of such toxicities, rigorous quantification and detailed characterization of CAR T-cell–derived cytokine release are indispensable for safeguarding therapeutic candidacy.
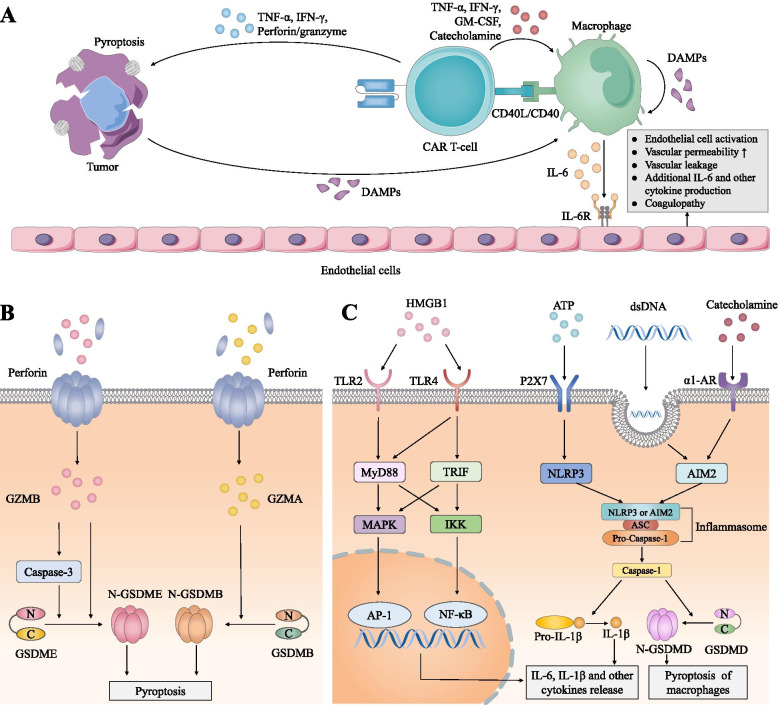 Fig.1 Key drivers of cytokine release syndrome.1
Fig.1 Key drivers of cytokine release syndrome.1
Creative Biolabs' CellRapeutics™ cytokine release test service focuses on the unique biological properties of γδ T cells and utilizes advanced technologies to engineer bespoke CAR-γδ T, which delivers accurate tumor targeting and potentiated therapeutic efficacy. Our service provides fully tailored design strategies coupled with rigorous in vitro and in vivo validation, accelerating your research from concept to clinical candidate.
Creative Biolabs offers a comprehensive portfolio of highly sensitive and robust assays designed for the precise quantification of cytokine release, which is essential for evaluating the safety and efficacy profiles of CAR-T cell therapies across all stages of the development pipeline.
Our CellRapeutics™ service follows a meticulous, multi-step workflow designed for clarity and scientific rigor.
Required Starting Materials:
Key Steps Involved:
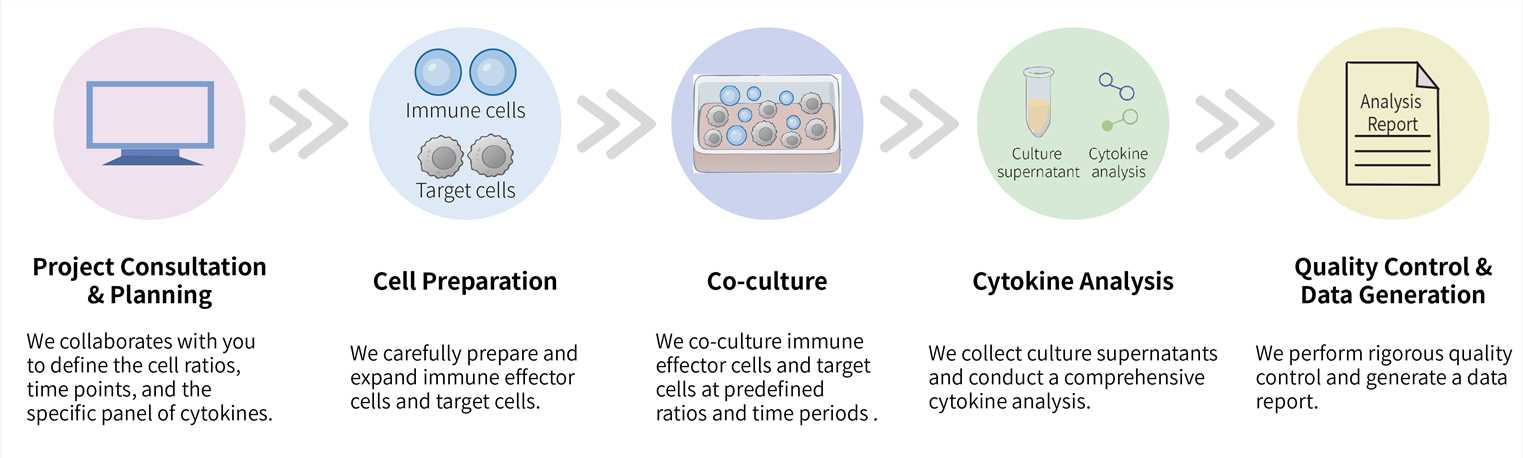
Final Deliverables:
Which cytokines are included in your detection panel?
Our standard panel encompasses pivotal pro-inflammatory mediators such as IL-6, IFN-γ, TNF-α, and IL-1β. In addition, we provide an expanded array covering over fifty cytokines, chemokines, and growth factors. Moreover, we also provide customized configurations aligned with specific research objectives.
How does your in vitro assay predict in vivo safety?
Our in vitro platform serves as a robust predictive model by recapitulating immune–target cell interactions under controlled conditions, which enables early identification of candidates with a propensity for inducing hyperinflammatory responses. While not a substitute for in vivo validation, the assay functions as an effective preclinical screening tool, facilitating the early exclusion of high-risk candidates.
As a leader in preclinical safety assessment, Creative Biolabs has advanced technology and extensive expertise, delivering a comprehensive cytokine release test service. Our robust testing platform generates reliable and reproducible data, empowering you to make confident and critical go/no-go decisions throughout your development process.
"Using Creative Biolabs' CellRapeutics™ Cytokine Release Test Service in our research has significantly improved our ability to predict potential clinical toxicities. The detailed analysis of IL-6, IFN-γ and IL-10 levels gave us the confidence to move our candidate forward, avoiding the pitfalls we'd seen with other labs." Jm P**er.
"Creative Biolabs' platform provided a side-by-side cytokine profile that clearly demonstrated our product's lower inflammatory potential. The data was clear, concise, and incredibly useful for our regulatory submission." Aa M*n.
"The detailed analysis from Creative Biolabs helped us understand the underlying mechanism of a previously observed off-target effect and allowed us to adjust our molecular design. The technical support was outstanding and provided key recommendations for our project's success." Ty W*s.
Creative Biolabs serves as your trusted scientific partner to address the complexities of cell therapy development. Our CellRapeutics™ Cytokine Release Test Service represents a critical element for ensuring both success and safety in your program.
To learn more about how our service can support your specific project or to request a detailed quotation, please contact our team using the link provided below.
Experience the Creative Biolabs Advantage - Get a Quote Today
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION

