By combining experienced immunotherapy solutions, streamlined process improvement, and cutting-edge technologies, Creative Biolabs provides integrated, science-driven, drug development services to enable our clients to achieve their drug discovery goals. We are dedicated to offering rigorous scientific expertise to ensure the most accurate and high-quality results.
In recent years, cancer immunotherapies have witnessed impressive success. The most effective and representative immunotherapies include immune checkpoint inhibition, T cell engaging therapies and chimeric antigen receptor (CAR) T cell therapies. However, along with infusion of these immunotherapies in the clinical application, fatal adverse effects, such as cytokine release syndrome (CRS) and neurotoxicity syndrome were observed. The toxicities are life-threaten for these immunotherapies. To support the successful application of innovative cancer immunotherapies, it's vital to manage the CRS to maintain under the safe range. Hence, Creative Biolabs launched a series of solutions to offer a broad range of drug discovery and development services to manage the CRS.
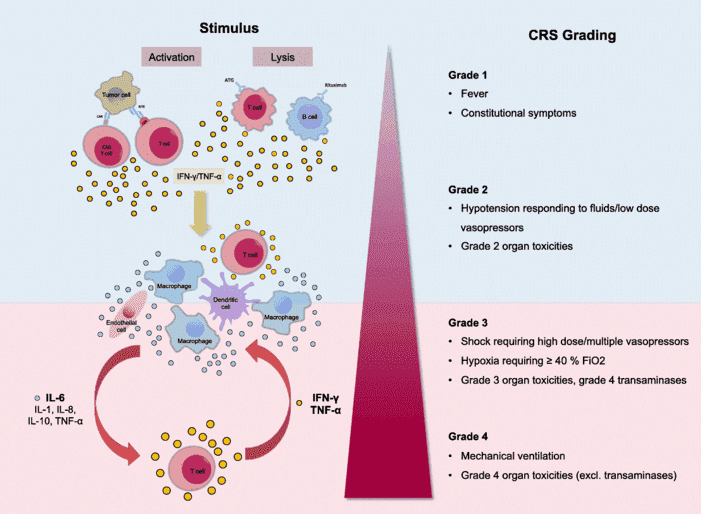 Fig.1 Proposed pathomechanism of CRS.1
Fig.1 Proposed pathomechanism of CRS.1
CRS is a symptom complex associated with various immunotherapies, and it's caused by excessive release of cytokines during activation of T cells and other immune cells. Major CRS-related cytokines include IL-6, IL-10, TNF-α, C-reactive protein (CRP), granulocyte-monocyte colony-stimulating factor (GM-CSF) and IFN-γ which will contribute to the toxicities. To control the adverse effects caused by CRS, and to enhance the clinical safety of immunotherapies, drug discovery and development for CRS management is necessary. One of the most valuable approaches is targeting cytokines themselves. At Creative Biolabs, we provide comprehensive drug discovery and development solutions for cytokine targeting approaches for every stage - all the way from early basic research to post-IND stage. To navigate through our full spectrum of services, please visit any of the categories below.
IL-6 has been identified as a significant cytokine in exacerbating the progression of CRS in T cell-related immunotherapies. Creative Biolabs is experienced in antibody drug discovery and development. As a value-added CRO with a focus on antibody discovery, engineering, and manufacturing, we offer end-to-end therapeutic antibody discovery services for our clients based on IL-6/IL-6R blockade.
It has been demonstrated that GM-CSF is related to the severity of CRS in clinical trials. Studies have indicated that the GM-CSF blockade can decrease the CRS and neuroinflammation. Creative Biolabs provides therapeutic antibody discovery services to develop novel drugs based on GM-CSF blockade, resulting in reduced CRS caused by immunotherapies.
IL-1 produced by macrophages has been put forward as a potential principal of neurotoxicity in immunotherapies. Creative Biolabs will help our clients develop novel IL-1R blockade therapeutics to attenuate CRS and neurotoxicity syndromes while keeping the efficacy of immunotherapies.
Creative Biolabs offers one-stop drug discovery and development for GSDME blockade approaches to manage the cytokine release syndrome caused by immunotherapies.
As a flexible partner in the complex process of drug discovery and development for CRS management caused by immunotherapies, Creative Biolabs provides integrated drug discovery services for every stage of the development - all the way from earlier discovery research to later IND application. Please feel free to contact us to learn more and have further communication.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION