Nanoparticles engineered to replicate DC properties ensure efficient antigen presentation and immune priming.
Dendritic cells (DCs)are the most powerful antigen-presenting cells, capable of priming both CD4+ and CD8+ T cells. DC-mimetic nanoparticles are designed to replicate this unique biology, offering stable antigen loading, controlled release, and precise targeting. Recent studies confirm that nanocarriers dramatically enhance vaccine efficacy, checkpoint blockade synergy, and tumor microenvironment adaptability. This approach positions DC mimetics as transformative platforms in immunotherapy and vaccine development. Creative Biolabs' DC mimetic nanoparticle development service enables you to overcome these challenges by replicating DC functions, optimizing antigen presentation, and driving strong adaptive immune responses through advanced nanotechnology engineering.
Creative Biolabs provides end-to-end services tailored to specific research or clinical needs:
By replicating the functions of natural DCs, our DC mimetic nanoparticles ensure robust antigen presentation, stronger immune priming, and improved delivery efficiency. These platforms overcome instability and weak immunogenicity that often limit conventional vaccines or drugs. With Creative Biolabs, clients gain access to a complete development pipeline—from design to functional validation—that accelerates discovery and enhances translational success.
Experience Tailored Support from Creative Biolabs – Book a Consultation
Choose from lipid-based, polymeric, inorganic, or hybrid nanoparticles engineered to mimic DC properties.
Employ optimized encapsulation methods to protect and deliver cargos with stability and controlled release.
Apply DC-mimetic coatings, ligands, or immune-targeting molecules to ensure specific immune engagement.
Assess particle size, charge, antigen loading, release kinetics, and surface marker expression.
Test DC uptake, antigen presentation, cytokine induction, and T cell activation in vitro.
Conduct efficacy studies in relevant disease models with comprehensive reporting and next-step recommendations.
Nanoparticles engineered to replicate DC properties ensure efficient antigen presentation and immune priming.
Capable of encapsulating proteins, peptides, nucleic acids, or multi-antigen payloads for diverse applications.
Integration with CpG and other immune stimulants boosts cytokine release and T cell activation.
Nanoparticles designed to reprogram the tumor microenvironment and synergize with checkpoint inhibitors.
Validated processes ensure batch-to-batch consistency and readiness for preclinical translation.
Discover the Creative Biolabs Edge – Start Your Quote Request
The schematic illustrates how biomimetic nanoparticles can be integrated into DC vaccination to enhance tumor immunotherapy. In this approach, nanoparticles are engineered to encapsulate tumor antigens and immune adjuvants, ensuring their stability and controlled release. Once administered, they interact with DCs, facilitating efficient antigen uptake and processing. The activated DCs then migrate to lymphoid tissues, where they present the processed antigens to T cells, initiating a strong cytotoxic response against tumor cells. By mimicking the natural functions of immune components, biomimetic nanoparticles improve targeting precision, prolong antigen availability, and reduce systemic side effects. This strategy not only amplifies the efficacy of DC vaccines but also creates opportunities for combination therapies that overcome tumor-induced immune suppression and achieve durable antitumor immunity.
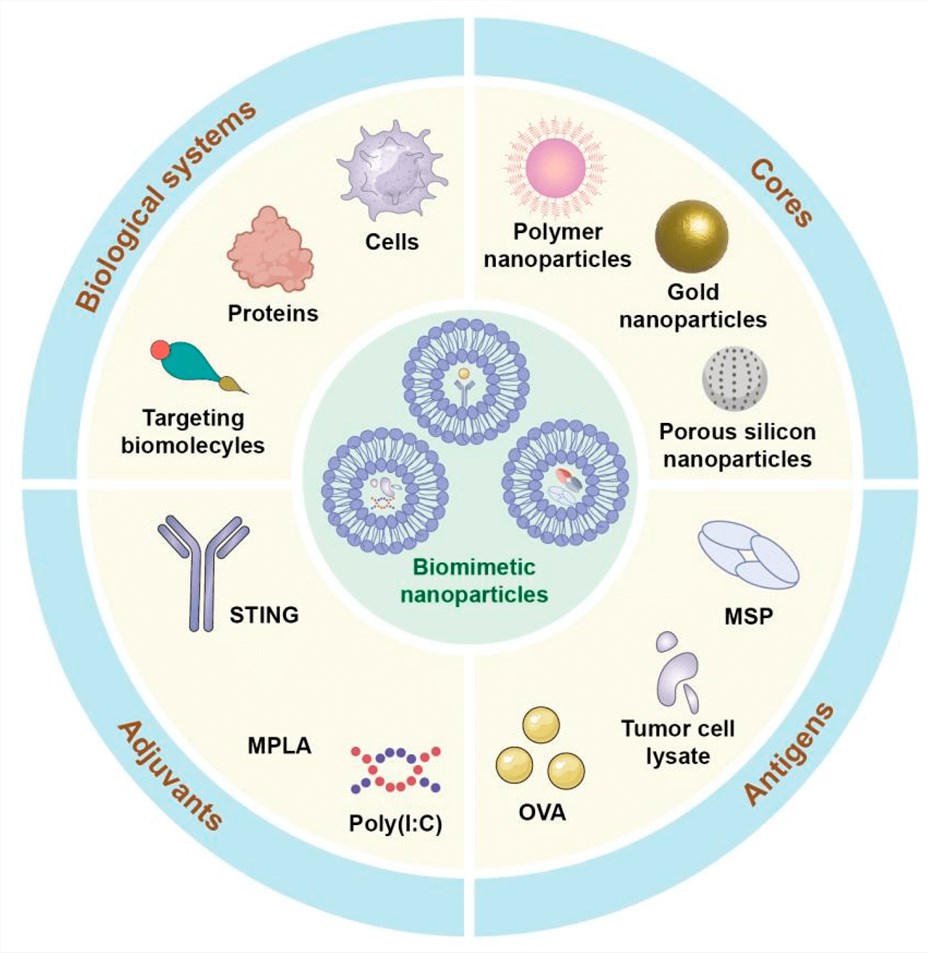 Fig.1 Illustrative overview of a DC–based vaccination strategy employing biomimetic nanoparticles.1
Fig.1 Illustrative overview of a DC–based vaccination strategy employing biomimetic nanoparticles.1
"Enhanced Antigen Stability: Using Creative Biolabs' DC-mimetic nanoparticles stabilized our peptide vaccine and significantly improved antigen persistence during in vivo studies." – [Dr. Ha**s]
"Superior T Cell Activation: Their nanoparticle platform provided stronger T cell responses than traditional adjuvant systems, helping us validate a novel immunotherapy concept." – [Prof. Ka**o]
"Streamlined Development: Creative Biolabs combined design, functional testing, and preclinical evaluation into a single workflow, saving us time and ensuring high-quality outcomes." – [Dr. Fe**r]
What types of antigens can be delivered with DC mimetic nanoparticles?
Our platforms can incorporate peptides, recombinant proteins, nucleic acids, or combinations tailored to project goals.
How do DC mimetic nanoparticles differ from standard nanocarriers?
They replicate antigen presentation and costimulatory functions of natural DCs, resulting in stronger immune responses.
How does Creative Biolabs ensure reproducibility?
We apply rigorous quality control, including physicochemical characterization and functional assays, to maintain consistency and scalability.
A service focused on improving delivery to and engagement with antigen-presenting cells—this aligns conceptually with nanoparticle strategies designed to mimic DCs and optimize antigen presentation.
While more focused on programming T cells, this service indicates experience in nanoparticle technologies for immune modulation—an adjacent capability that could integrate with DC-mimetic delivery systems.
Creative Biolabs delivers next-generation DC Mimetic Nanoparticle Development Services, ensuring precise antigen presentation, enhanced immune activation, and translational reliability. Our platforms provide an integrated pathway from design to preclinical validation.
Contact Our Team for More Information and to Discuss Your Project
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION