Donor-dependent in vivo mouse models provide indispensable value in cytokine profiling, as they enable dynamic and quantitative assessment of donor-specific risks such as CRS and immune activation status during treatment. Creative Biolabs' Donor-dependent In Vivo Mouse Model Development Service leverages a range of highly humanized models to establish an advanced platform that faithfully recapitulates human tumor-immune interactions. We are committed to delivering integrated and highly predictive evaluations of both immunotherapeutic efficacy and safety, thereby overcoming the limitations associated with conventional model systems.
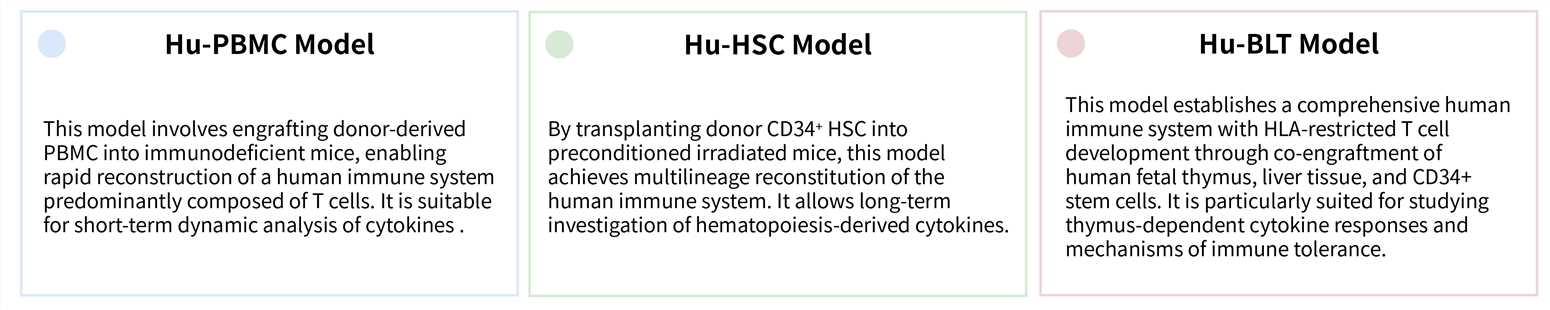
Donor-dependent in vivo mouse models are advanced preclinical platforms established by engrafting functional human immune cells from specific donors into immunodeficient mice, thereby recapitulating the human immune system within an in vivo setting to evaluate immunotherapies. These models primarily include the Hu-PBMC, Hu-HSC, and Hu-BLT systems, each distinguished by the source of immune cells and the resulting immune profile. A key advantage of such donor-dependent models lies in their capacity to provide integrated, donor-specific cytokine profiling, enabling precise assessment of both therapeutic efficacy and immune-related adverse events, such as CRS, thereby supporting more predictive and translatable immuno-oncology research.
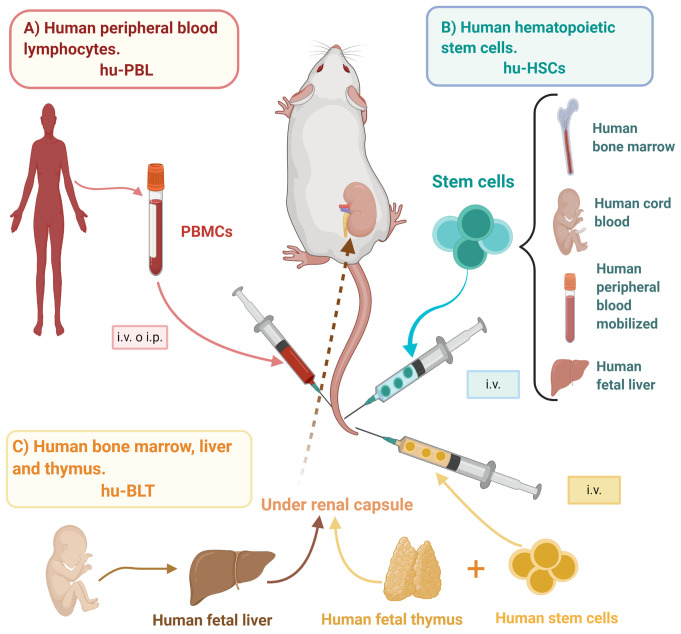 Fig.1 Development of donor-specific humanized mouse models for immuno-oncology research.1
Fig.1 Development of donor-specific humanized mouse models for immuno-oncology research.1
Creative Biolabs' Donor-dependent In Vivo Mouse Model Development Service utilizes a range of highly humanized mouse models to accurately recapitulate the complexity of the human immune system and its interactions with tumors. Our platform enables a systematic and quantifiable assessment of critical parameters during therapy, including CRS risk and anti-tumor efficacy, thereby delivering predictive preclinical data to support the efficacy and safety evaluation of immunotherapies.
We provide donor-specific immune cells to reconstitute murine models, enabling you to faithfully recapitulate the human immune microenvironment and establish a robust platform for cytokine profiling and analysis.
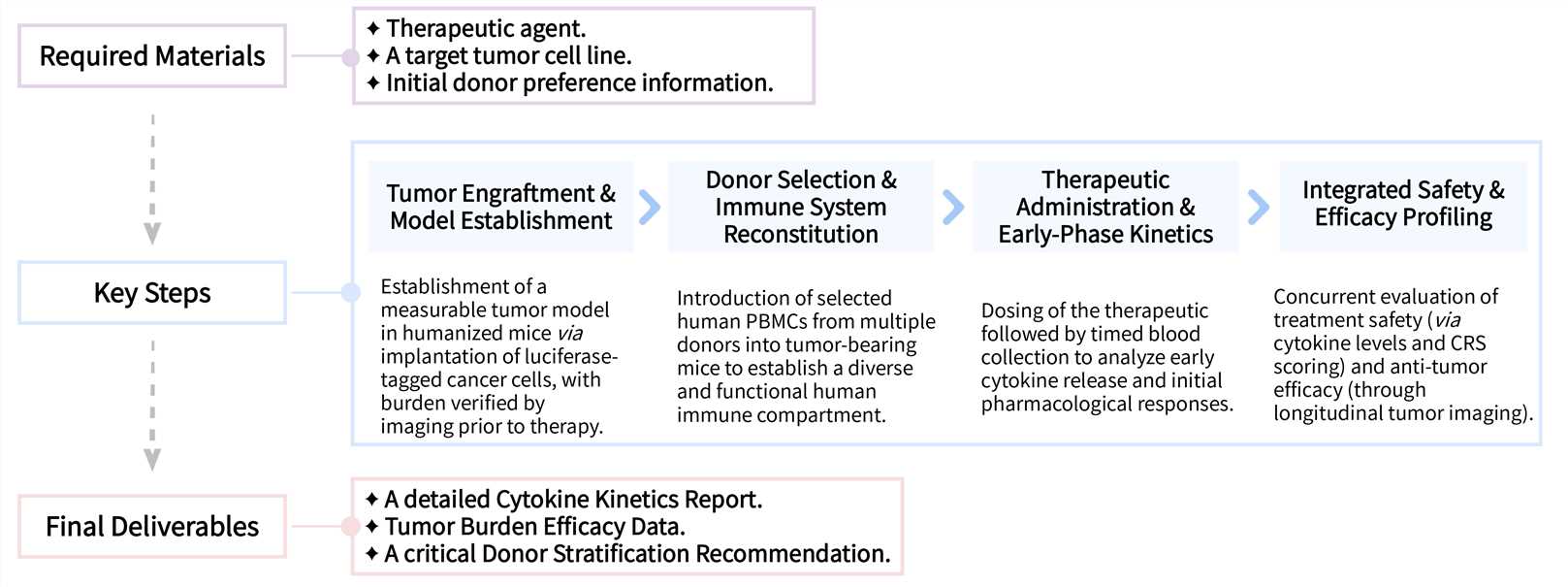
Creative Biolabs follows a transparent, meticulously planned workflow, ensuring clear expectations and timely project completion.
How do you select the PBMC donors to ensure donor-dependent variability is covered?
We maintain a proprietary library of characterized donors with documented high, medium, and low responsiveness to various immune triggers. We recommend testing your therapeutic against a panel of 3-5 diverse donors to ensure robust stratification and prediction of population variability.
Can this service be used to test combination therapies?
Of course. Our model is specifically optimized to evaluate combination strategies, allowing you to measure additive or synergistic CRS and efficacy responses when co-administering, for example, a CAR-T with an adjuvant.
As a global leader in in vivo toxicology and efficacy testing, Creative Biolabs possesses profound expertise in xenograft models and immuno-oncology. Our distinguished Donor-dependent In Vivo Mouse Model Development Service is uniquely designed to address key clinical challenges, including patient variability and combination therapy, by delivering exceptional predictive power. We go beyond conventional survival analyses to provide mechanistic insights that are critical for optimal candidate selection.
"Using Creative Biolabs' Donor-dependent In Vivo Mouse Model Development Service in our research has significantly improved our ability to distinguish between low- and high-risk therapy. The clear donor stratification data provided a level of predictability that traditional in vitro assays completely lacked, saving us months of development time."— Emi** R****.
"The dual imaging and cytokine analysis facilitated a true understanding of the link between tumor kill and CRS onset. The detailed data was instrumental in justifying our starting dose for our application, directly addressing our concerns regarding therapeutic window. Creative Biolabs' quick turnaround was essential."— Dr. H*ng L.
"We utilized this service to test our CAR-T and a novel immune-modulating drug combination. The ability to track synergistic cytokine release in vivo gave us the confidence to move forward with the combination regimen, something we couldn't achieve with co-cultures."— Feli** S****h.
Based on our donor-dependent in vivo mouse model development service, we deliver a dual, integrated assessment of both therapeutic efficacy and donor-specific CRS risk, enabling more informed decision-making and helping reduce the likelihood of clinical failure.
We invite you to contact our team for further information and to discuss your specific project requirements.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION