Navigating the complexities of CAR-T cell therapy development is challenging, especially when it comes to monitoring CAR-T cell kinetics, ensuring sample stability, and standardizing data across multi-site clinical trials. Creative Biolabs' Flow Cytometry Application in CAR-T System offers a solution to these hurdles. Our advanced multiparametric flow cytometry platforms are designed to streamline and accelerate your research by providing robust, reproducible data. This allows you to focus on the core scientific questions, moving your CAR-T therapy development forward with greater confidence and efficiency.
Flow cytometry, a laser-based optical technology, enables high-speed, multiparametric quantification and sorting of individual cells in suspension. In the discovery and clinical validation of CAR-T therapies, it has become indispensable: from phenotypic profiling and functional assessment of CAR-T cells, including activation status, exhaustion markers, and cytokine secretion, to longitudinal monitoring of in vivo and ex vivo cell kinetics and global immune status throughout treatment. By standardizing quality control of CAR-T products and furnishing robust biomarkers of therapeutic response and durability, flow cytometry has markedly enhanced the controllability and safety of CAR-T immunotherapy.
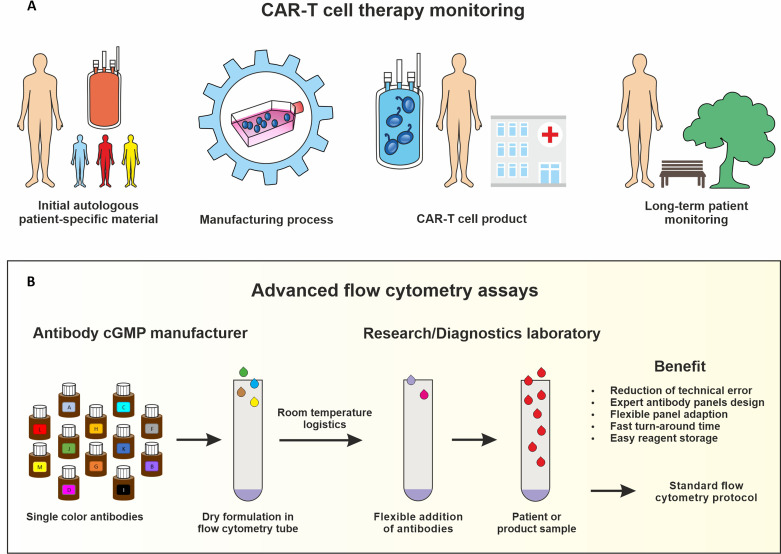 Fig.1 Application of advanced flow cytometry in CAR-T cell therapy monitoring.1
Fig.1 Application of advanced flow cytometry in CAR-T cell therapy monitoring.1
Creative Biolabs' Flow Cytometry Application in CAR-T System provides a fully integrated portfolio of flow-cytometry services engineered exclusively for CAR-T cell discovery and patient-tailored surveillance. Leveraging ultra-high-sensitivity, dual-parameter-optimized panels, we enumerate CAR-T cells, resolve their phenotypic landscape, and map their longitudinal persistence and biodistribution in vivo, delivering the quantitative, decision-grade data required to advance programs from bench to bedside with confidence.
Creative Biolabs integrates a comprehensive suite of flow cytometry platforms throughout the entire CAR-T therapy development pipeline from preclinical candidate screening to clinical-phase patient monitoring. This systematic application ensures rigorous quality control, guaranteeing that the final cellular product is characterized by high viability and desired biological function.
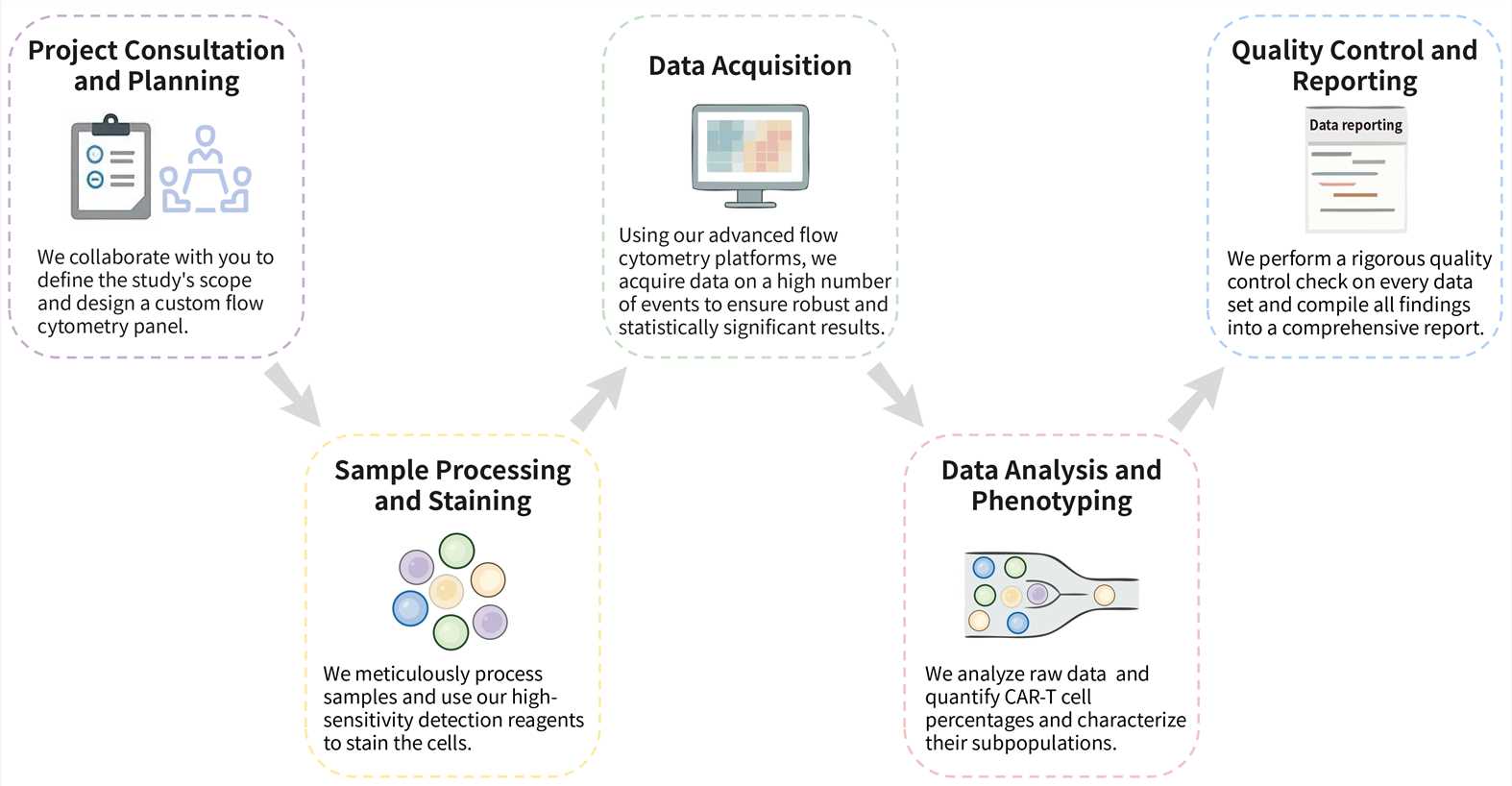
We ensure a streamlined and collaborative experience in applying flow cytometry to your CAR-T projects. Through proactive communication and meticulous attention to detail, our process translates your specific requirements into actionable, high-fidelity data, de-risking your path to critical milestones.
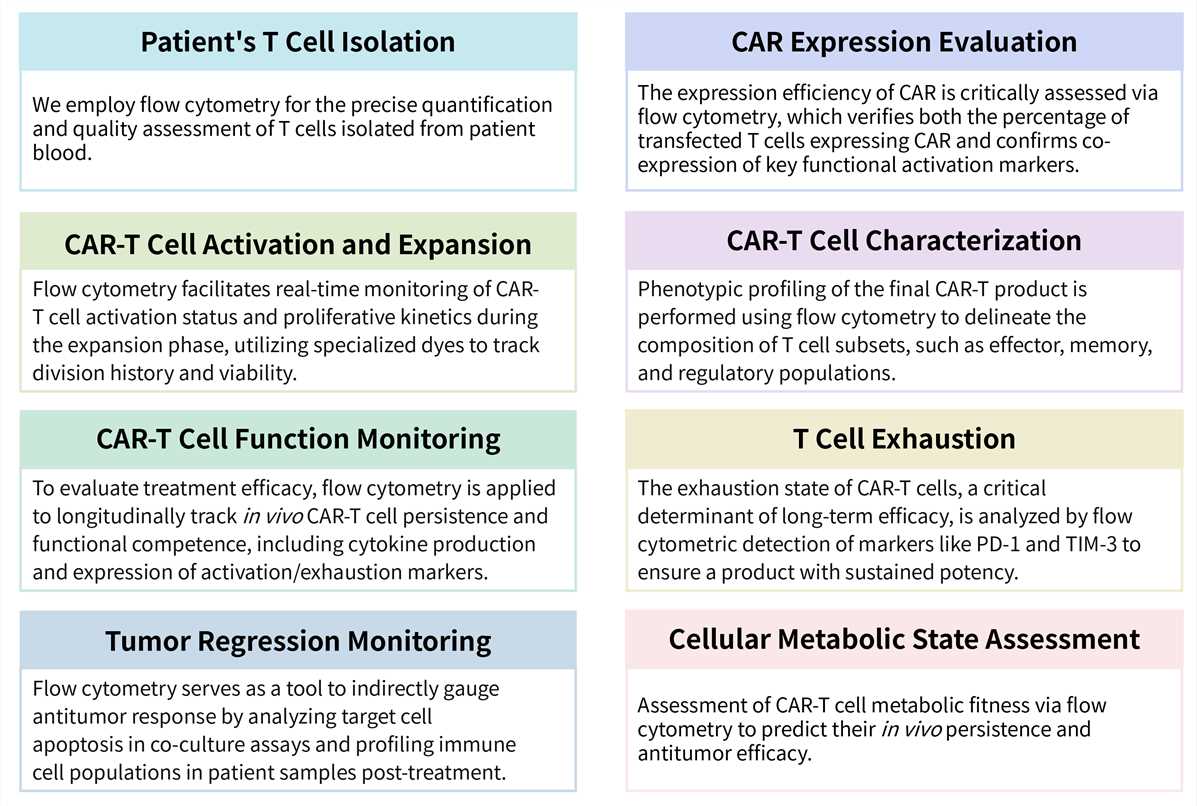
Required Starting Materials:
Key Steps Involved:
Final Deliverables:
What is the principal advantage of employing your flow cytometry service for CAR-T cell monitoring compared to traditional qPCR methods?
While qPCR provides quantitative data on transgene presence, our flow cytometry platform delivers a critical functional dimension. Its principal advantage lies in providing multiparametric, precision resolution in a significantly shorter turnaround time. This enables simultaneous assessment of not only cell count and viability but also critical functional phenotypes.
Is your service capable of supporting the analysis of next-generation CAR-T constructs, such as those engineered for multi-antigen targeting?
Yes, definitively. Our flow cytometry assays are inherently adaptable and are rigorously validated for characterizing advanced CAR-T constructs, including tandem (dual-targeting) CARs and those utilizing bicistronic vectors. We specialize in developing custom antibody panels to precisely identify, quantify, and functionally characterize these sophisticated multiclonal cell populations.
As a premier expert in antibody discovery and immunotherapy, Creative Biolabs offers comprehensive, end-to-end solutions for CAR-T therapy development. Flow cytometry serves as an indispensable laser-driven analytical tool for evaluating CAR-T cell properties, spanning applications from biomarker detection to clinical-stage validation. Based on our integrated CAR-T service platform, Creative Biolabs provides a full suite of flow cytometry instruments and reagents designed to ensure precise and reliable outcomes.
"The application of Creative Biolabs' flow cytometry solutions has been instrumental in achieving profound phenotypic characterization of our CAR-T cells. The exceptional sensitivity of their detection reagents enabled us to accurately quantify even minimal populations of circulating CAR-T cells, a critical factor for assessing patient-specific therapeutic responses." D. J***son, Principal Scientist.
"Creative Biolabs' services delivered the level of precision required for our clinical-stage work. The high degree of correlation established between their flow cytometry analyses and our orthogonal qPCR data provided strong validation, allowing us to confidently adopt their more efficient platform for routine monitoring." M. M***ler, Clinical Research Manager.
"The operational expertise from Creative Biolabs was transformative. They collaborated with us to optimize a sample-handling protocol that effectively mitigated stability concerns during inter-facility transportation, thereby safeguarding data integrity and avoiding significant project delays." A. G***a, Lab Director.
Our consortium of specialists is prepared to collaboratively engage with your team to design and implement a customized strategy, precisely aligned with your unique project requirements. We welcome you to initiate a conversation with us to facilitate an in-depth discussion.
Leverage Our Expertise – Discuss Your Project and Receive a Quote
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION