Cytokine release syndrome (CRS) is an acute systemic inflammatory syndrome associated with various therapies including, gene therapy. Creative Biolabs offers the most extensive portfolio of cytokine analysis platforms and services for measuring CRS-associated cytokines biomarkers and evaluating the CRS process. Our cytokines assays can be summarized as a comprehensive set of methods and protocols, including bioassays, protein microarrays, sandwich enzyme-linked immunosorbent assay (ELISA), high-performance liquid chromatography (HPLC), bead based multiplex immunoassays, and so forth.
Gene therapy is understood as the capacity for gene improvement using the correction of altered genes or site-specific modifications that have treatment as a target. One of the most frequently used techniques consists of recombinant DNA technology, in which the gene of interest or healthy gene is inserted into a vector. Among different types of vectors, the viral vector is the most often used due to its efficiency in invading cells and introducing its genetic material. Depending on the target cell type, the gene therapy is currently subdivided into two large groups: gene therapy of somatic cells and gene therapy of the germline. Gene therapies can work by the following techniques: gene augmentation therapy, gene inhibition therapy, and killing of specific cells.
Gene therapy is normally conducted by the introduction of new techniques, such as induced pluripotent stem cells (iPSC) in combination with current models of genetic editing, and even by trials in germ cells. TCR-gene therapies are also part of the emerging treatment landscape secondary to the common underlying principle of immune effector cell activation causing tumor cell death. Meanwhile, CRS represents a potentially serious complication of gene therapy. The main cytokines elevated in the pathogenesis of gene therapy associated CRS include interleukin-1 (IL-1), IL-2, IL-2Rα, IL-6, IL-8, IL-10, interferon (IFN)-γ, monocyte chemoattractant protein 1 (MCP-1), granulocyte-macrophage colony-stimulating factor (GM-CSF), and tumor necrosis factor (TNF).
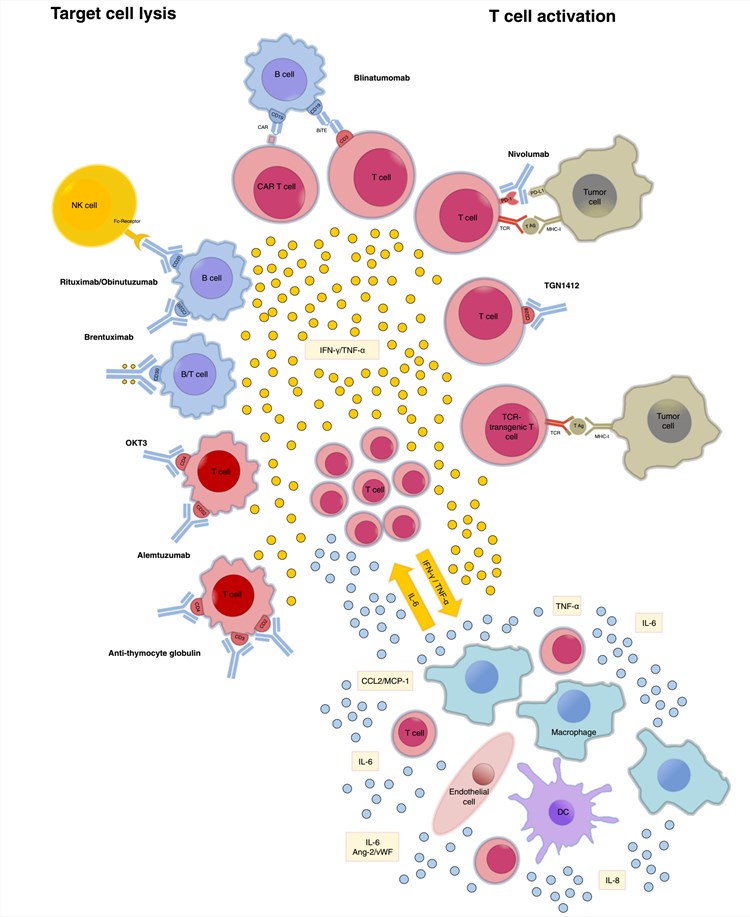
Fig.1 Reported inducers of CRS.1
The recommendations for the management of gene-therapy-related CRS are still evolving constantly. Fig.1 shows the reported inducers of CRS. For any interested cytokine, in vitro or in vivo cytotoxicity analysis and cytokine services could be put to good use. For instance, the observation that IL-6 is elevated in the serum of patients with CRS following gene therapy has led some investigators to consider anti-IL-6 or anti-IL6R as a rapid resolution of CRS symptoms. Anti-cytokine antibody and CRS model development services could be supplied to global clients.
It can be expected that the treatment algorithms for CRS will change in the future as Creative Biolabs gain more and more experience with managing the side effects of gene therapy. If you are interested in our services, please contact us for more information.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION