Creative Biolabs offers a genetically engineered DC development service that utilizes advanced DNA construct pulsing and precise genetic modulation to enhance immune responses, overcome immunosuppressive barriers, and facilitate potent, targeted immune activation for various immunotherapy applications.
Dendritic cells (DCs) are pivotal antigen-presenting cells (APCs) that bridge the innate and adaptive immune responses. Despite their therapeutic promise, traditional DC-based immunotherapies often yield limited clinical success due to challenges in antigen loading efficiency, stability, and the pervasive immunosuppressive tumor microenvironment (TME). Genetic engineering, particularly through DNA construct pulsing, offers a revolutionary approach to enhance DC function, enabling precise manipulation for superior antigen presentation and overcoming TME-induced suppression.
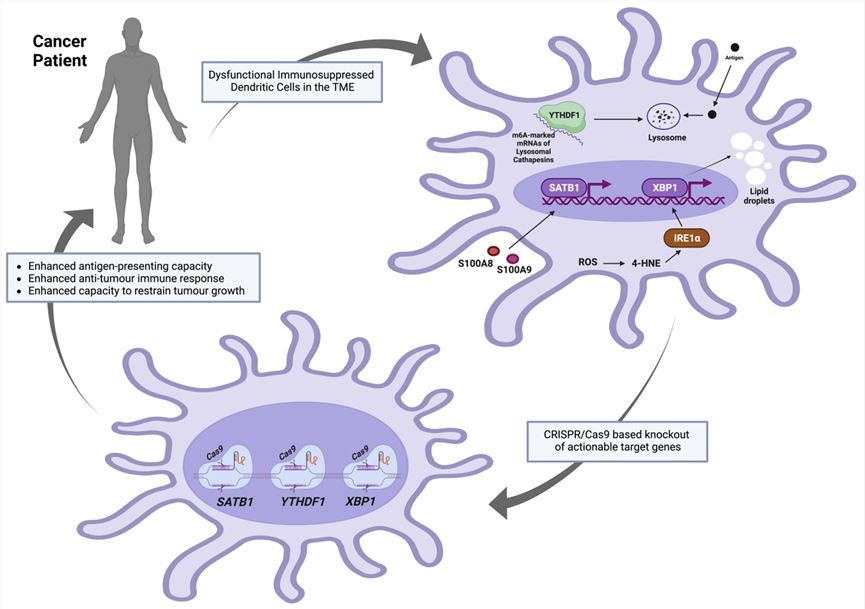 Fig.1 Gene editing methods are used to knock down the target genes in DCs.1
Fig.1 Gene editing methods are used to knock down the target genes in DCs.1
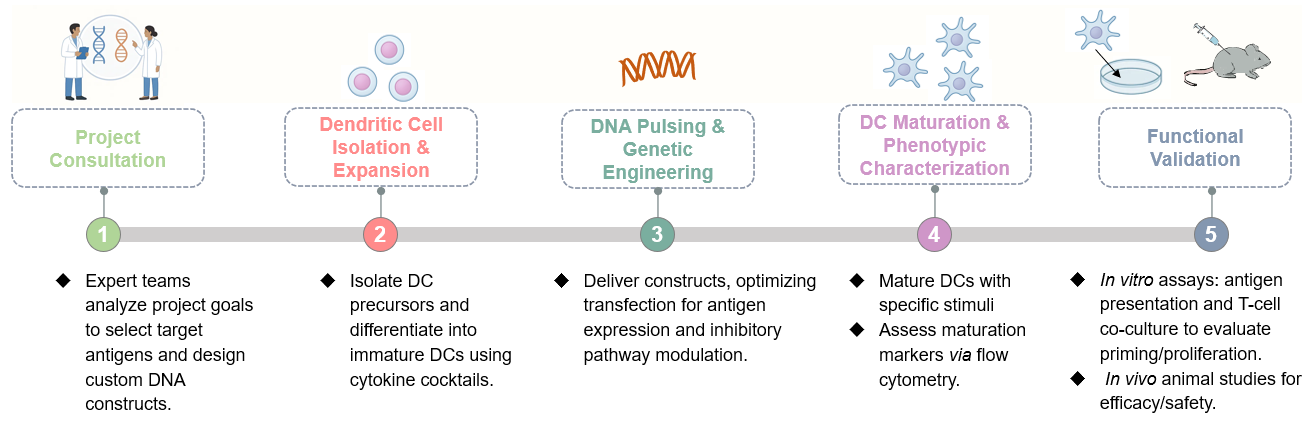
Creative Biolabs' genetically engineered DC development service delivers advanced immunotherapy solutions by pulsing dendritic cells with optimized DNA constructs, ensuring targeted antigen expression and potent immune activation. Leveraging precision transfection methods—such as electroporation, viral vectors, or nucleofection—the approach achieves high-efficiency gene delivery without compromising viability or function. The genetically engineered DCs exhibit robust antigen presentation, enhanced maturation, and co-stimulatory molecule expression, fueling strong T-cell priming and durable immunity essential for therapeutic and translational applications.
We provide resolutions to:
| Optimized Nucleic Acid Design and Synthesis |
|---|
|
| High-Yield DC Isolation and Culture |
|---|
|
| Advanced Non-Viral Gene Delivery Systems |
|---|
|
| Precise Genetic Modulation Capabilities |
|---|
|
| Comprehensive Functional Characterization and Validation | |
|---|---|
|
|
Q1: What types of antigens can your DNA Construct Pulsing service deliver?
A1: Our service is versatile, delivering tumor-associated antigens, neoantigens, and pathogen-specific ones. Using DNA/mRNA constructs, we can handle various protein sequences and design fusion proteins for better immunogenicity. Consult with our scientists for your specific antigen needs.
Q2: How does ex vivo DC engineering differ from in vivo LNP-mRNA administration?
A2: Ex vivo engineering offers advantages. It enables precise control over DC maturation and antigen loading, reduces systemic LNP exposure and related risks, and allows thorough DC characterization before re-administration, ensuring a potent cellular vaccine.
Q3: Can you target specific DC subsets for immunotherapy?
A3: Yes. We can isolate and differentiate subsets. We'll select and engineer the most suitable subset for your therapeutic goal. Contact us to explore our DC subset capabilities.
Creative Biolabs stands as a pioneer in advanced cell-based immunotherapies, offering unparalleled expertise and a commitment to accelerating your research. Our genetically engineered DC development service is distinguished by key advantages and unique features.
Looking to learn more about our genetically engineered DC development service and how it can propel your research forward? Our expert team is available to discuss your project and provide tailored guidance.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION