Welcome to Creative Biolabs' Good Manufacturing Practices (GMP) grade virus manufacturing services designed specifically for CAR-T therapies. Our state-of-the-art facility is fully compliant with GMP, ensuring that we produce high-quality viral vectors that meet regulatory standards. We specialize in the production of lentiviruses, adenoviruses, and other viral vectors essential for the development of cutting-edge cell therapies. With a dedicated team of experts and a commitment to innovation, we provide tailored solutions that streamline the manufacturing process and support your research trials, enabling you to advance your CAR-T therapies with confidence.
GMP Grade Virus refers to viral preparations that are produced according to Good Manufacturing Practices (GMP). These practices ensure that biological products, including viruses, are consistently produced and controlled in a quality manner suitable for their intended use, particularly in clinical trials and therapeutic applications.
GMP-grade viruses are essential for various applications, including:
GMP standards cover various aspects of production, including the quality of starting materials, the production environment, monitoring and testing for contaminants, documentation, and traceability. Meeting these standards helps to ensure that the final product is safe for use in humans
Creative Biolabs is excited to offer our specialized GMP Grade Virus Manufacturing service for CAR-T therapies. Our state-of-the-art facility is equipped to produce high-quality viral vectors under stringent GMP regulations, ensuring that every batch meets the rigorous standards required for clinical applications. Our team of experienced scientists and engineers is dedicated to optimizing the production process, providing you with reliable and scalable solutions tailored to meet your specific project needs. By partnering with us, you can be confident in the quality and efficacy of your CAR-T products, facilitating advancements in personalized immunotherapies.
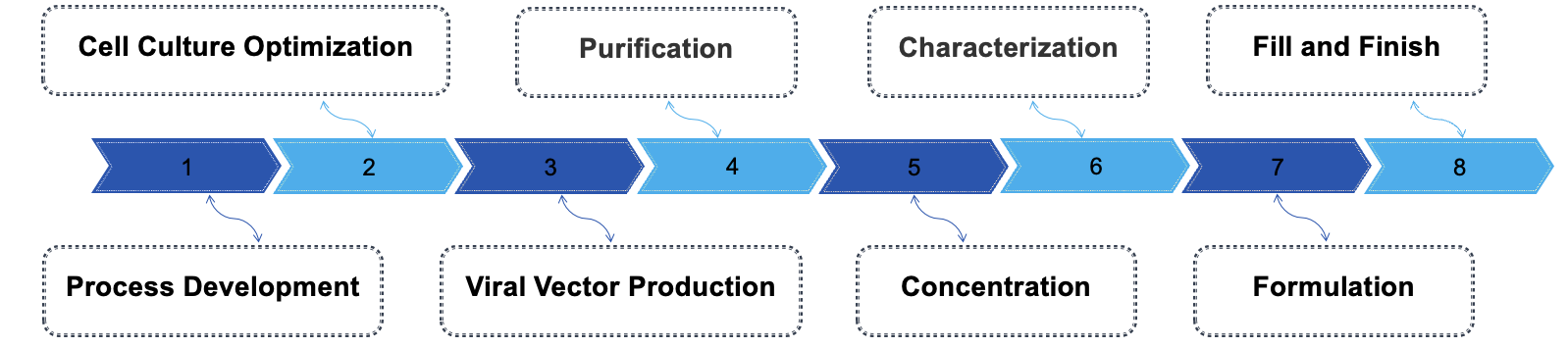
The production of GMP-grade virus vectors used in CAR T therapy involves several critical steps to ensure safety, quality, and efficacy. Firstly, the process begins with the selection of suitable viral vectors, often derived from lentiviruses or retroviruses, followed by the design of the CAR construct. Once the vector is engineered, stringent quality control measures are employed during the cell culture phase, where producer cell lines are maintained under controlled conditions to ensure consistent yields of viral particles. Following this, purification processes, such as ultracentrifugation or chromatography, are implemented to isolate the viral particles from cellular debris and other contaminants. Each batch undergoes rigorous testing for potency, identity, and sterility to meet regulatory standards.
We provide a comprehensive quality control framework to ensure viral vectors are safe, effective, and stable. The following table summarizes the main testing categories and typical release criteria:
| Test Category | Technology | Typical Release Criteria | Notes |
|---|---|---|---|
| Titer Measurement | Titer Measurement |
Lentivirus: ≥1×109 TU/mL Retrovirus: ≥1×109 TU/mL AAV: ≥1×1013 GC/mL |
Ensures sufficient infectious units for intended use |
| Replication Competence | RCL / RCV assays | Negative (limit of detection ≤1 TU/mL) | Confirms absence of replication-competent virus |
| Integration Site Analysis | LAM-PCR / NGS | Random distribution without abnormal hotspots | Evaluates insertional safety |
| Residual Host Proteins | ELISA / SDS-PAGE | ≤50 ng/mL | Reduces potential immunogenicity |
| Residual Host DNA | qPCR | ≤10 ng/dose | Minimizes the risk of unwanted DNA |
| Endotoxin | LAL assay | ≤5 EU/mL | Ensures product safety for injection |
| Purity / Impurity Analysis | SDS-PAGE / HPLC | ≥90% target component | Ensures batch consistency and purity |
| Stability Studies | Storage at -80°C / -20°C / 4°C, accelerated conditions | Retains ≥80% initial titer over 3 months | Supports long-term storage and transport |
Discover How We Can Help - Request a Consultation.
What production scale and titer can you achieve?
We offer flexible scales from early-phase to Phase II/III. Titer depends on your construct, but our platform delivers highly concentrated vectors for efficient CAR-T transduction. Specific goals can be discussed in consultation.
How is RCV risk controlled?
Through RCV-free producer cells, controlled GMP environments, and validated RCV release assays for every lot, ensuring maximum safety.
Can you use proprietary plasmids?
Yes, if produced under GMP-like or GMP standards with full documentation. We can also manufacture plasmids in-house for immediate compliance.
Do you support other delivery systems like AAV or mRNA?
Core expertise is Lentivirus and Adenovirus for CAR-T/TCR-T, but we also offer AAV and mRNA/LNP platforms. Contact us to discuss alternative vector needs.
Using Creative Biolabs' GMP CAR-T Virus Manufacturing Service in our research has significantly improved/facilitated the quality of our CMC documentation, making our IND submission process seamless and stress-free. La Do.
Using Creative Biolabs' GMP CAR-T Virus Manufacturing Service in our research has significantly improved / facilitated our final cell transduction rate, offering a vector that was demonstrably higher in infectious titer and lower in host cell DNA compared to our previous CRO's product. An Pl.
We are ready to help you with your GMP-grade virus manufacturing needs. For any inquiries, quotes, or to discuss your specific requirements, please feel free to contact us. You can reach us via email at info@creative-biolabs.com. Additionally, feel free to fill out our online contact form on our website, and we'll get back to you promptly. Let us partner with you in advancing your CAR-T therapies through high-quality viral vector production.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION