GMP, or Good Manufacturing Practice, comprises a series of regulations and guidelines designed to guarantee the safety, quality, and effectiveness of pharmaceutical products, including biologics such as cell and gene therapies. CAR-T therapy entails the modification of a patient's T-cells to enhance their ability to target and eradicate cancer cells effectively. The manufacturing of CAR-T cells is a complex process that often requires the production of viral vectors to efficiently deliver the CAR genes into the T-cells. At Creative Biolabs, we specialize in GMP-compliant Virus Manufacturing for CAR-T cell therapy, providing cutting-edge solutions that ensure the highest standards of quality, safety, and efficacy.
Good Manufacturing Practice (GMP) standards for CAR-T (Chimeric Antigen Receptor T-cell) therapies are a set of rigorous regulations and guidelines implemented to ensure the quality, safety, and efficacy of these advanced cell therapies. CAR-T therapies involve genetically modifying a patient's own T-cells to recognize and attack cancer cells. Due to the complexity and the personalized nature of these therapies, compliance with GMP is critical.
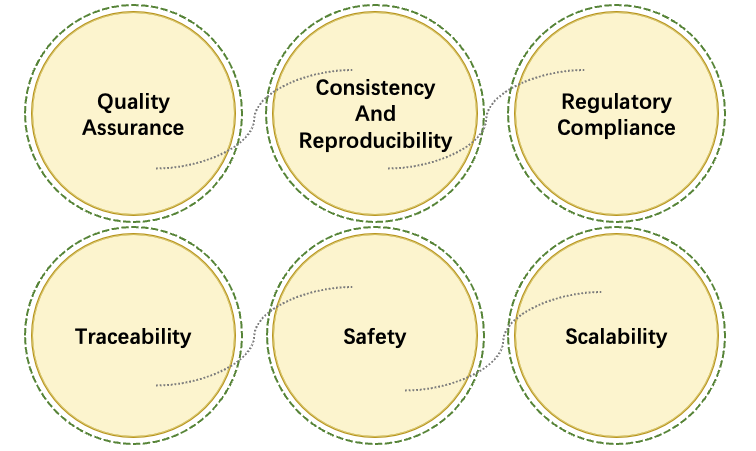
Our GMP VLP manufacturing services are designed to produce high-quality, compliant VLPs that serve as essential tools for the delivery and expression of CAR constructs in therapeutic applications. Utilizing state-of-the-art bioprocessing techniques, GMP VLP manufacturing ensures the scalability and reproducibility necessary for clinical-grade products. By adhering to stringent regulatory standards, these services offer a reliable platform for vector development, enabling researchers to effectively harness the potential of CAR-T cells in targeted cancer therapies. Furthermore, the use of VLPs enhances the safety and efficacy profile of CAR-T products, ultimately advancing the field of immunotherapy and improving patient outcomes.
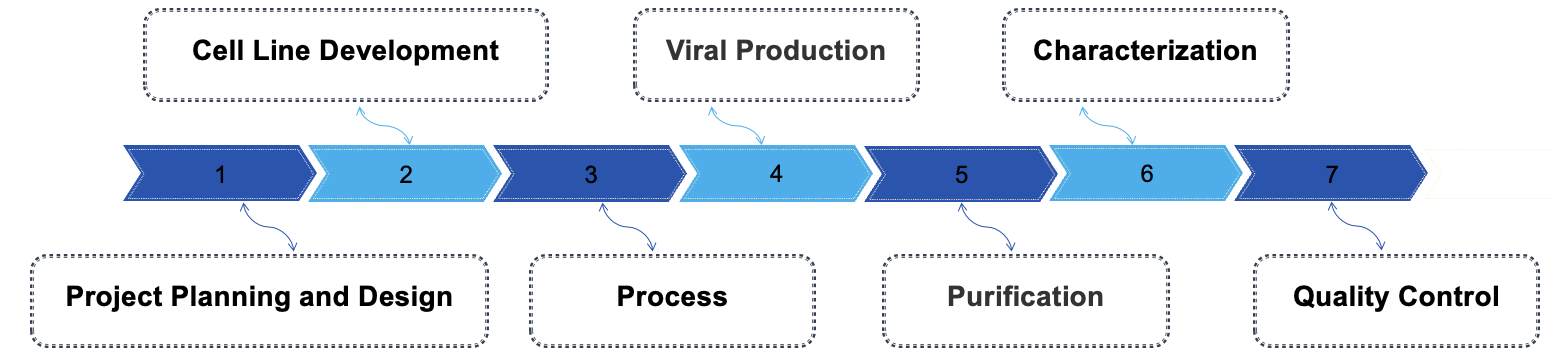
Our GMP-like virus manufacturing process encompasses several essential steps to guarantee the production of high-quality viral products. Firstly, the process begins with stringent planning and design, where the facility is tailored to meet regulatory standards, including clean room specifications and equipment validation. Next, the cell culture development phase is initiated, where suitable host cells are selected and optimized for viral replication. Following this, viral inoculation and propagation take place, necessitating closely monitored conditions to promote efficient viral growth while preventing contamination. Downstream processing is the subsequent stage, where the harvested virus is purified through methods like filtration and chromatography to achieve the desired purity and potency. Throughout the entire process, stringent quality control measures are enforced, including batch testing and stability evaluations, to ensure adherence to regulatory standards.
| Category | Assay Type | Analytical Methods | Purpose |
|---|---|---|---|
| Identity | Vector genome confirmation | qPCR, sequencing, restriction enzyme digestion | Confirm vector identity and integrity |
| Potency | Infectious titer / transduction efficiency | Flow cytometry, qPCR, functional reporter assays | Demonstrate functional vector activity |
| Purity | Residual host cell proteins | ELISA, Western blot | Detect impurities from producer cells |
| Residual host cell DNA | PicoGreen, qPCR | Quantify DNA impurities | |
| Residual plasmid DNA | qPCR | Assess process residuals | |
| Replication-competent virus | Cell-based amplification assay | Ensure vector replication incompetence | |
| Safety | Sterility & mycoplasma | Compendial USP <71> / <63> methods | Confirm absence of microbial contamination |
| Endotoxin | LAL assay | Ensure product safety | |
| Stability | Accelerated / real-time stability studies | Storage at controlled conditions | Define vector shelf life and storage parameters |
Discover How We Can Help - Request a Consultation.
What is the main difference between your "GMP-like" service and full "Clinical GMP" manufacturing?
GMP-like service provides high-quality vectors, full documentation, and strict QC testing using qualified equipment and trained personnel, ideal for early preclinical studies. Full GMP involves additional client-specific audits and long-term facility scheduling, which we can support when needed.
How do you guarantee the absence of RCV?
Safety is paramount. We use sensitive, validated assays—including co-culture amplification and molecular methods—to detect RCV. Every batch is tested to ensure safe, high-quality viral vectors.
Can your platform handle both Lentivirus and Retrovirus vector production and custom CAR constructs?
Yes. Our flexible, closed-system platform efficiently produces both lentiviral and retroviral vectors. We can accommodate a wide range of CAR constructs, including advanced signaling domains, with vector optimization to maximize yield and quality.
How do you prevent cross-contamination between client batches?
We use single-use, closed-system technologies and segregated cleanroom suites. Rigorous cleaning and validated changeover procedures ensure no cross-contamination between campaigns.
Using Creative Biolabs' GMP-like CAR-T Virus Manufacturing Service in our research has significantly improved the purity of the final transduced T-cell product, leading to more reproducible and reliable in vitro functional assays. The low residual HCP count was critical for our in vivo studies. - (Dr. J**es).
We faced persistent issues scaling our own vector production. Creative Biolabs' high-density bioreactor platform solved this, delivering a titer 5x higher than our previous internal benchmark, significantly reducing the cost per dose for our upcoming Phase I trial. - (Dr. B***r)
Creative Biolabs' state-of-the-art facility is equipped with the latest technologies and adheres to stringent regulatory protocols to produce viral vectors tailored to meet the specific needs of our clients. From research and development to full-scale production, our experienced team is dedicated to supporting researchers and biopharmaceutical companies in their pursuit of innovative therapies. Contact us to accelerate your CAR-T projects with reliable and compliant virus manufacturing services. Together, let's propel the future of CAR-T therapy forward!
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION