Chimeric antigen receptor (CAR) T-cell therapy has emerged as a breakthrough approach in cancer immunotherapy, especially for hematological malignancies. However, there are many challenges for the CART treatment in solid tumors, including on-target, off-tumor toxicity and antigen heterogeneity. A key aspect in the development of safe and effective CAR-T cell therapies lies in the precise identification of target epitopes. Epitope mapping helps to understand antibody-target interactions and select optimal lead candidates. High-throughput epitope mapping technologies are accelerating this process, enabling the rapid screening of CAR-T cell candidates and enhance the development of CAR-T cell therapies.
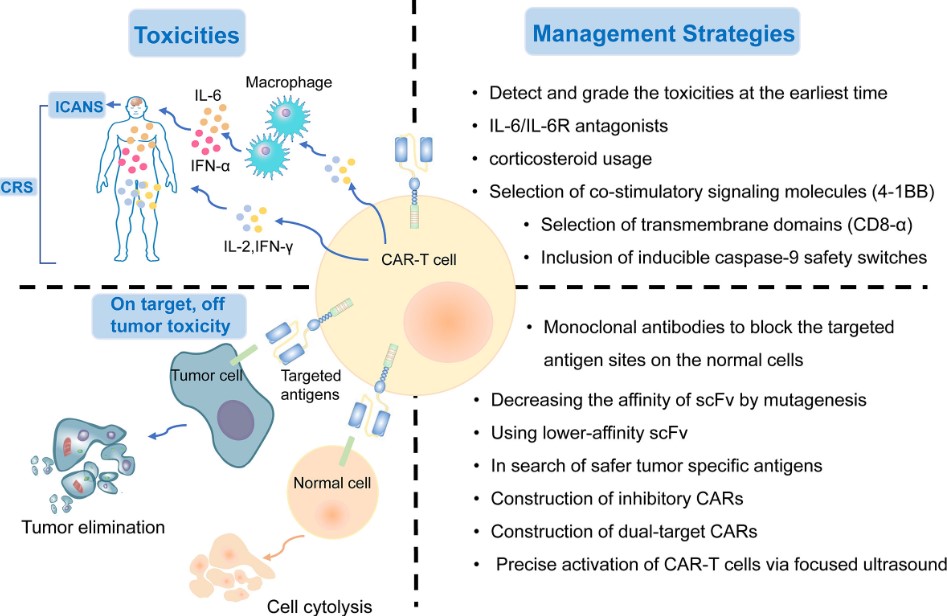 Fig.1 Toxicities and management strategies of CAR-T therapy.1
Fig.1 Toxicities and management strategies of CAR-T therapy.1
Creative Biolabs offers a cutting-edge high-throughput epitope mapping service designed to accelerate and enhance the development of functional CAR-T cell therapies. This service leverages advanced technologies to provide rapid, high-resolution epitope mapping, facilitating the identification of optimal CAR designs with enhanced specificity and efficacy. Our service addresses the critical need for efficient screening methods in the CAR-T cell therapy development pipeline, enabling researchers to overcome key challenges associated with on-target, off-tumor effects and antigen variability.
Creative Biolabs' High-Throughput Epitope Mapping Service offers a comprehensive suite of features designed to meet the demanding requirements of CAR-T cell therapy development:
Our core technology is based on massive parallel mutagenic scanning, enabling the simultaneous analysis of a large number of CAR-T cell candidates. This approach dramatically accelerates the epitope mapping process, providing results in a fraction of the time compared to traditional methods.
The service provides high-resolution epitope mapping, allowing for the precise identification of critical binding sites. This level of detail is essential for understanding antibody-target interactions and designing CAR-T cells with optimal binding affinity and specificity.
Our service is capable of running up to hundreds of candidates in parallel. This high-throughput capability significantly increases efficiency and reduces the time required for screening.
Our service is designed to integrate seamlessly with functional CAR-T cell screening workflows. This integration allows for the rapid evaluation of CAR-T cell activity, ensuring that selected candidates exhibit not only high binding affinity but also potent effector functions.
We understand that each CAR-T cell development project is unique. Creative Biolabs offers customizable solutions tailored to the specific needs of our clients, ensuring that our service provides the optimal level of support and expertise.
Q1: What types of CAR-T cell constructs are compatible with your epitope mapping service?
A1: Our service is compatible with a number of CAR-T cell constructs, including those based on various scFv formats and CAR designs. We can accommodate both humanized and fully human antibodies.
Q2: How long does the epitope mapping process take?
A2: The turnaround time for our High-Throughput Epitope Mapping Service is significantly faster than traditional methods. The exact duration depends on the specific project requirements and the number of CAR-T cell candidates being analyzed. We will do our best to establish timelines that meet their research needs.
Q3: What level of data analysis and reporting is provided?
A3: We provide comprehensive data analysis and reporting, including detailed maps of identified epitopes, binding affinities, and other relevant parameters. Our reports are designed to be clear, concise, and easy to interpret, enabling our clients to make informed decisions about their CAR-T cell development programs.
Creative Biolabs is committed to providing customers with the highest quality epitope mapping services and expert support. If you have any questions about our High-Throughput Epitope Mapping Service, please do not hesitate to contact us.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION