Solid-phase assays have been introduced to distinguish complement binding from non-complement binding human leukocyte antigen (HLA)-specific antibodies. Solid-phase C1q binding assay can detect the ability of bead-bound HLA antibodies to fix complement and detect the products of complement activation, such as C3d, C4d, and C1q. Creative Biolabs provides HLA single antigen-C1q screening to characterize the potentially high titer or complement activating antibodies.
The HLA single antigen-C1q screening is the improvement of the HLA single antigen class I and class II bead test that detects the fixation of C1q, the first component of the classical complement cascade, to anti-HLA antibodies bound to beads. This test enables characterization of potentially high titer or complement activating antibodies and the specificities of C1q binding antibodies are reported.
The C1q assay aims at defining HLA antibodies that can bind C1q. Firstly, serum samples are tested by the C1q assay which is heat-inactivated to eliminate the interference of endogenous complement components. Secondly, sera are incubated together with recombinant C1q, after which binding of both antibody and C1q is detected by a PE-labeled anti-C1q antibody (shown as Fig.1). Since there is no selection for IgM or IgG, C1q-binding HLA antibodies detected can be either IgM or IgG isotype. Creative Biolabs also provides modified versions of this assay, such as anti-human globulin enhancement or two-step detection of C1q-binding HLA antibodies which can increase sensitivity.
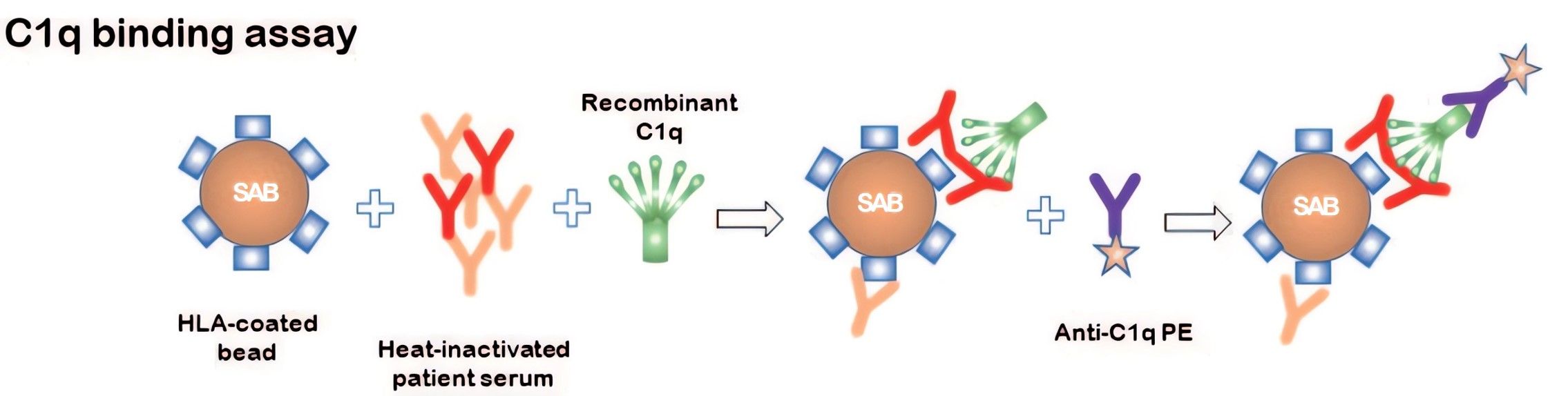 Fig.1 Principle of C1q binding assay.1
Fig.1 Principle of C1q binding assay.1
The main purpose of pre-transplant HLA antibody testing is to define unacceptable antigens to which strong, complement-fixing antibodies are present in the recipient. Evidence has shown that if complement-binding characteristics of pre-transplant donor-specific antibodies (DSA), such as C1q and IgG3, have the ability to stratify patients into high and low risk for allograft loss, administration of pre-transplant complement-targeting therapies could help transplantation of particularly highly sensitized patients having these kinds of DSA.
When focusing on C1q positivity, the patients with C1q+ DSA had a five-year graft survival of only 54%, which indicates that C1q positivity increases the chance of inferior graft survival. In a study on acute antibody-mediated rejection (ABMR) treatment by plasmapheresis, scientists found that posttreatment conversion of DSA from C1q+ to C1q- showed better specificity and positive predictive value to predict graft survival as well as response to therapy than a reduction of ≥50% in IgG DSA MFI.
With in-depth development and advanced laboratory equipment in HLA research, Creative Biolabs offers a full range portfolio of assays for low- to high-resolution detection. We also offer the most cost-effective multiplex assays and a wide range of singleplex kits. For more information, please feel free to contact us directly.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION