The service goes beyond general toxicity screens by measuring hematopoietic functionality, lineage potential, and stemness maintenance under immune challenge.
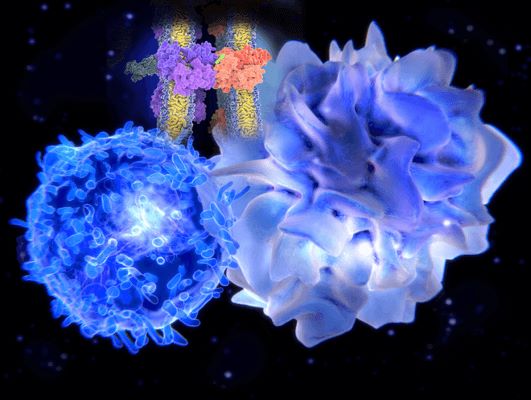
As immune effector cell (IEC) therapies such as CAR-T and CAR-NK continue to expand across cancer immunotherapy pipelines, a recurring challenge has emerged in the form of prolonged cytopenias and impaired hematopoietic recovery. Termed Immune Effector Cell-associated Hematotoxicity (ICAHT), this phenomenon reflects more than transient myelosuppression—it indicates sustained disruptions to hematopoietic stem/progenitor cells (HSPCs), their differentiation potential, and the regulatory bone marrow microenvironment.
The pathogenesis of ICAHT is complex, involving multifactorial biological processes: inflammatory cytokine exposure, direct or bystander cytotoxicity, oxidative stress, immune-mediated niche remodeling, and exhaustion of hematopoietic precursors. Despite increasing clinical awareness, the mechanistic basis of ICAHT remains poorly understood, underscoring the need for controlled, research-grade systems to dissect these pathways preclinically.
To address this need, Creative Biolabs has developed the ICAHT Mechanism Research Service—a high-resolution platform designed to decode the fundamental biological processes that drive immune-mediated hematotoxicity. This service enables researchers to model hematopoietic dysfunction in vitro, assess cellular phenotypes and viability under immune effector pressure, and identify the molecular cascades associated with hematopoietic suppression and recovery failure.
Our ICAHT Mechanism Research Service offers an integrated and customizable experimental workflow, focusing on mechanistic discovery and functional characterization of hematopoietic suppression induced by IECs. This service is compatible with a broad range of effector cell formats (CAR-T, CAR-NK, TCR-T) and allows for the evaluation of construct-specific, activation-induced, or cytokine-driven toxicity profiles.
Quantitative colony-forming unit (CFU) assays (CFU-GM, BFU-E, CFU-Mix) to evaluate progenitor differentiation potential under IEC-conditioned environments.
Flow cytometry and imaging-based readouts for apoptosis (Annexin V, Caspase-3), senescence (β-Gal), and necrosis in hematopoietic populations.
Exposure of CD34⁺ cells to CAR-T or cytokine-rich supernatants (e.g., IFN-γ, TNF-α, IL-1β) to assess indirect hematopoietic inhibition.
RNA-seq, ATAC-seq, and methylation profiling to explore signaling pathways, lineage commitment, and epigenetic dysregulation.
In vitro reconstruction of bone marrow microenvironments using stromal cell lines or primary MSCs to mimic immune-mediated niche injury.
Measurement of ROS production, mitochondrial depolarization, and nutrient depletion in HSPCs during immune effector exposure.
These mechanistic tools enable researchers to correlate immune activation profiles with hematopoietic outcomes, distinguish between reversible and persistent suppression patterns, and identify early biomarkers of hematopoietic vulnerability.
The service goes beyond general toxicity screens by measuring hematopoietic functionality, lineage potential, and stemness maintenance under immune challenge.
Users can simulate early post-infusion inflammation, chronic cytokine exposure, or overactivation via CAR co-stimulation (e.g., 4-1BB vs CD28).
Combined use of molecular, cellular, and metabolic assays allows precise mapping of immune-driven hematopoietic dysfunction.
Compatibility with human CD34⁺ HSPCs and stromal cells ensures translational value, especially when combined with primary effector cells.
Seamlessly integrates with other Creative Biolabs services (e.g., cytokine detection, immunophenotyping, genomic analysis) to build a full ICAHT profile.
Ideal for side-by-side benchmarking of different IEC designs or optimization of constructs for reduced hematopoietic impact.
Q1: Can I compare multiple IEC constructs using the same assay conditions?
A1: Yes, we can run parallel assays under standardized conditions for comparative analysis.
Q2: What if I want to test immune suppression via cytokine exposure only?
A2: We offer cytokine-conditioned assays using recombinant cytokines or effector cell supernatants, with optional neutralization experiments.
Q3: Is it possible to include omics profiling?
A3: Absolutely. RNA-seq, proteomics, and epigenetic profiling can be incorporated into any assay module.
The ICAHT Mechanism Research Service is designed to empower discovery-focused teams working to understand and overcome hematopoietic toxicity in immune cell therapy. Whether you're developing next-gen CAR constructs or investigating immune-host interface biology, Creative Biolabs offers deep expertise and advanced platforms to accelerate your goals.
We welcome partnerships with biotech innovators, academic labs, and translational research groups seeking customized support and long-term collaboration. Let us help you generate actionable insights into one of the most critical safety aspects of cell-based immunotherapy.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION