The rapid development of immuno-oncology (IO) therapies for multiple types of cancer has transformed the cancer treatment landscape and brightened the long-term outlook for many patients with advanced cancer. With unrivaled expertise and experiences in cytokine release syndrome (CRS) research, Creative Biolabs independently developed a novel generation of CRS analysis platform to manage the severe CRS caused by IO. Our scientists are dedicated to various mechanisms to discover innovative and sought-after approaches for precision medicine with CRS management capacity.
Compared with traditional cancer therapies, the IO is emerging as a novel approach to cancer treatment through the stimulation of the body's immune system. The IO therapy offers a more effective treatment alternative for some patients with cancer. IO therapies generally engage the immune system to recognize and eradicate tumor cells. Key features of immune-mediated therapy include breadth of response, specificity, and memory. Several types of immunotherapy are used to treat cancer including immune checkpoint inhibitors (ICIs), T-cell transfer therapy, monoclonal antibodies (mAbs), treatment vaccines, and immune system modulators. Among them, ICIs have had remarkable success across multiple malignancies and are the most well-established IO agents to date, with several approvals. However, the unique kinetics and properties of immunotherapy also result in different incidence and types of side effects. Currently, the two main challenges for IO agents are affording the high cost of these novel therapies and managing their toxicities.
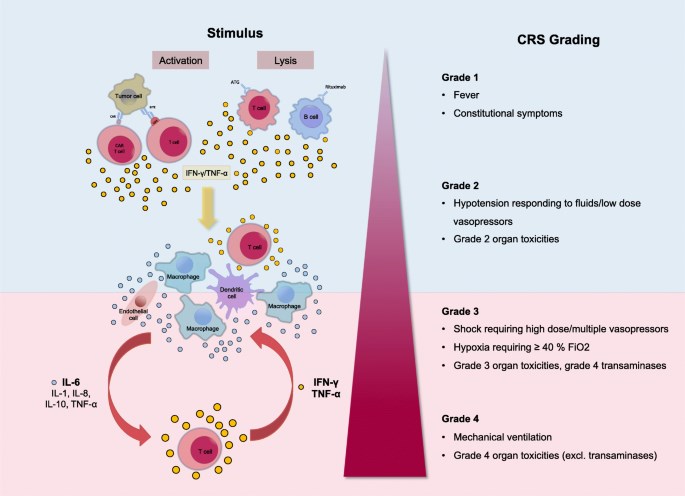 Fig.1 Proposed pathomechanism of CRS.1
Fig.1 Proposed pathomechanism of CRS.1
CRS is a collection of symptoms that can develop as a side effect of certain types of immunotherapy, especially those which involve T-cells. CRS occurs after treatment with immunotherapy that activates T cells to fight cancer. It was identified as an early potentially lethal clinical syndrome for IO therapy, but an effective clinical management strategy was quickly identified, which did not appear to interfere with efficacy.
While the field of IO therapy has advanced significantly in the past several decades, much more knowledge is needed to achieve a future where the potential benefit of these therapies can be maximized. Key questions remain about:
For Creative Biolabs, management of CRS includes comprehensive laboratory tests from cell level analysis to in vivo CRS model development. We offer in vitro cytokine release assay service by FCM, ELIspot, and ELISA. In vitro cytotoxicity analysis services are also available for you. Customized CRS research model could be tailored according to your specific requirement.. Please contact us for more information about the different assays or services that are available to support your programs.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION