Exploring innovative strategies to enhance the efficacy of CAR-T cell therapy for solid tumors has been favored by researchers. One such strategy involves the combination utilization of mRNA liposome pulsed dendritic cells (DCs) and CART solution. mRNA-based vaccines have gained attention for their ability to deliver antigen-encoding messages, thereby stimulating the immune system to generate a targeted immune response. In this context, the combination of mRNA liposomes with DCs provides a platform for efficient antigen presentation to CAR-T cells, leading to enhanced immune activation and tumor eradication.
As a leading biotechnology company specializing in advanced immunotherapy research, Creative Biolabs offers innovative CART development strategies and comprehensive in vitro CAR-T cell functional analysis under specific stimulation. Through these comprehensive analytical services, we assess the activation, proliferation, and cytotoxicity of CAR-T cells upon exposure to DCs loaded with mRNA liposomes encoding target antigens. By analyzing key functional parameters, such as cytokine secretion profiles and target cell killing efficiency, we provide detailed insights into the potency and specificity of CAR-T cell responses in vitro.
Our team utilizes a variety of cell proliferation assays to assess the growth and expansion of CAR-T cells in vitro under specific stimulation. Techniques such as flow cytometry-based assays, CFSE dilution assays, and MTT assays are employed to monitor cell proliferation over time and under different experimental conditions.
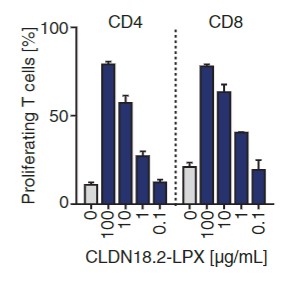 Fig.1 Proliferation test of CD4+ or CD8+ CAR-T cells analyzed by flow cytometry.1
Fig.1 Proliferation test of CD4+ or CD8+ CAR-T cells analyzed by flow cytometry.1
The assessment of the ability of CAR-T cells to kill target cells is a crucial aspect in predicting the efficacy of CAR-T cell therapy against cancer. This analysis involves evaluating the cytotoxic potential of engineered T cells against specific target antigens. Creative Biolabs offers comprehensive approaches for assessing cytotoxicity including Chromium-51 release assay, flow cytometry-based assays, LDH-based assay, and real-time cell analysis (RTCA).
Measurement of cytokine release by CAR-T cells is crucial for assessing their functional activity and immune response. Our experts conduct cytokine release assays to analyze the production of key cytokines such as IFN-γ, IL-2, and TNF-α, providing valuable insights into the efficacy of CAR-T cell therapy with mRNA Liposome pulsed DCs.
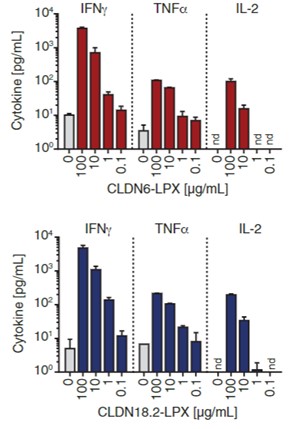 Fig.2 Cytokine test of CAR-T cells co-culture with claudin-expressing DCs by a multiplex assay.1
Fig.2 Cytokine test of CAR-T cells co-culture with claudin-expressing DCs by a multiplex assay.1
At Creative Biolabs, we specialize in providing cutting-edge services for CART development with innovative strategies. Our customized approach to this innovative field ensures that our clients receive bespoke solutions tailored to their specific requirements.
With years of experience in the field, Creative Biolabs has a deep understanding of designing CAR molecules for various targets, including mRNA-based approaches.
Our in vitro and in vivo pharmacodynamic experimental platforms are well-established, allowing for a thorough characterization of CAR-T cell functionality post mRNA liposome pulsed DC interaction.
Understanding that every project is unique, we offer fully customizable services tailored to meet the specific requirements and objectives of each client. Our team of experts collaborates closely with clients to ensure that the provided solutions align perfectly with their goals.
By choosing Creative Biolabs for your CART development, you can benefit from our expertise, proven track record, and commitment to delivering high-quality, customized solutions. Contact us to learn more about how our services can advance your research and development efforts in the field of CAR-T therapy.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION