In vitro transcribed mRNA CAR-T (IVT mRNA CAR T) cells act as a safe tumor therapy capable of addressing off-target tumor toxicity, in which T cells are briefly reprogrammed with mRNA encoding chimeric membrane antigen receptor proteins to combat TSA or TAA. In addition, IVT-mRNA CAR-T reduces side effects associated with off-target tumor toxicity due to mRNA instability. Currently, with the development of in situ cell engineering technology, in vivo IVT mRNA-based CAR-T engineering has been gradually developed for cancer immunotherapy.
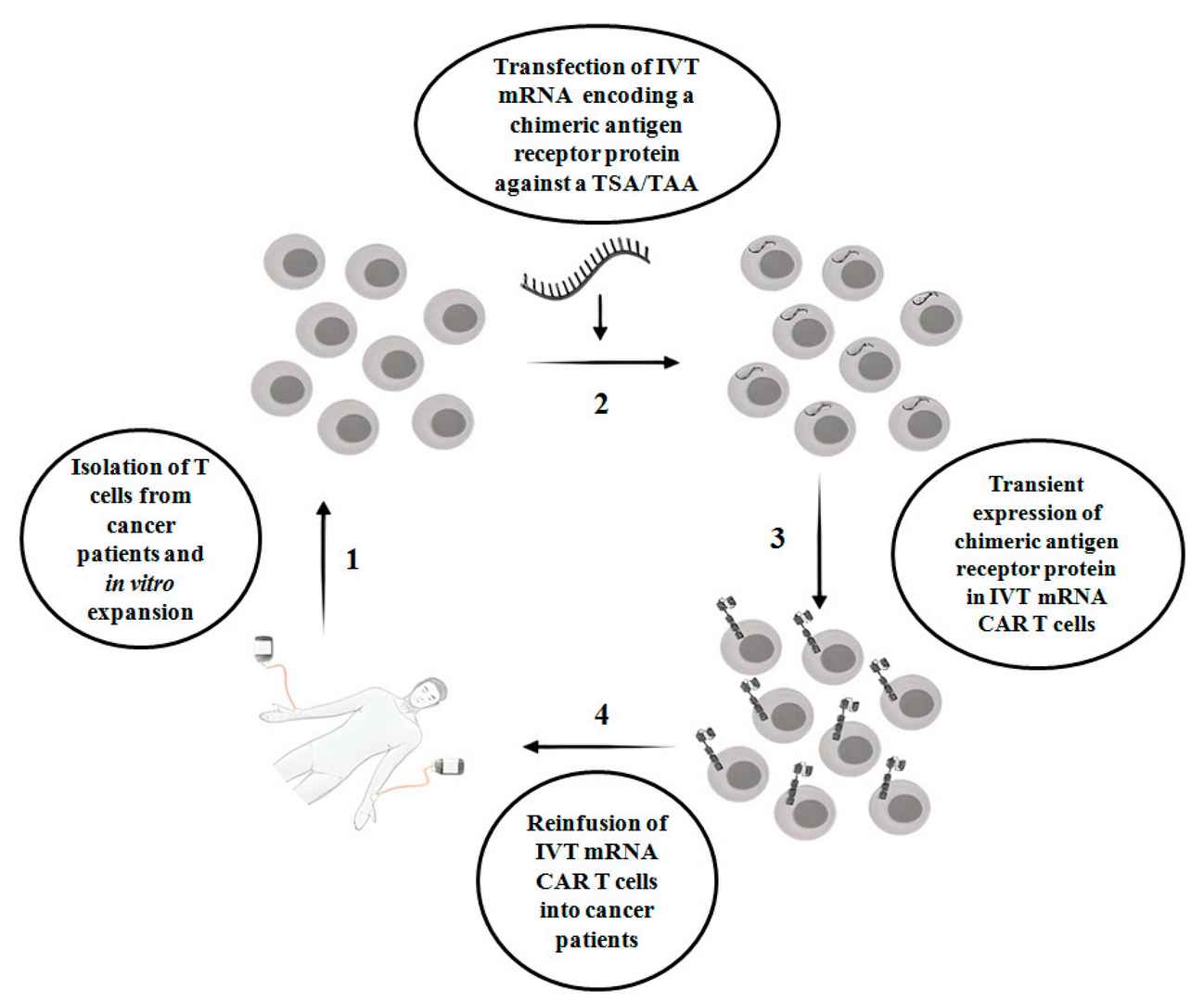 Fig.1 IVT mRNA CAR-T therapy strategy. (Soundara Rajan, 2020)
Fig.1 IVT mRNA CAR-T therapy strategy. (Soundara Rajan, 2020)
Creative Biolabs is dedicated to providing a safe and effective in vivo IVT mRNA-based CAR-T engineering service for to speed up global customers’ projects. Since IVT mRNA CAR-T cell therapy can drive away the tumor immune responses and on-target off-tumor response in both hematologic and solid tumors, we launch in vivo IVT mRNA-based CAR-T engineering by modifying the IVT mRNA structure and selecting the appropriate CAR domain. Regarding the service, we provide a one-stop service to meet customer needs, including but not limited to CAR design, IVT mRNA optimization & production.
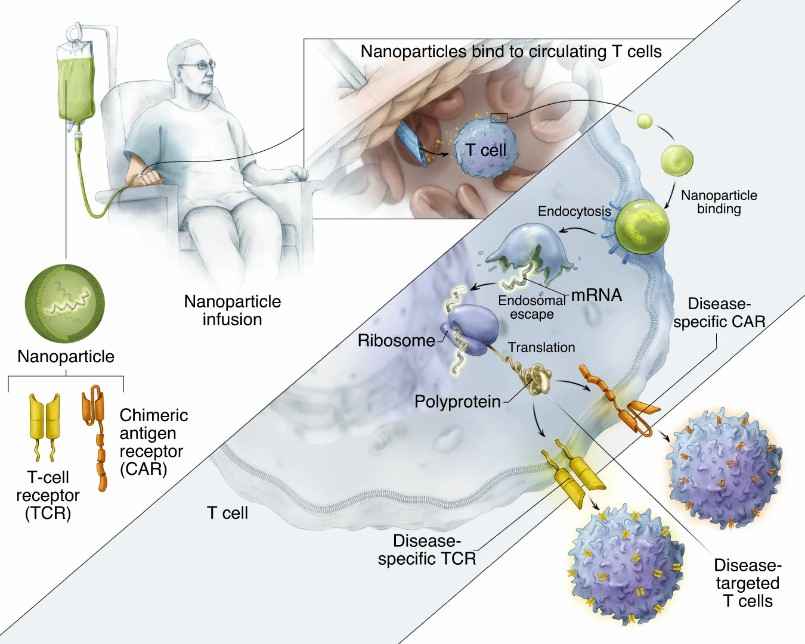 Fig.2 IVT mRNA transported by polymeric nanoparticles programming T cells in situ. (Parayath,2020)
Fig.2 IVT mRNA transported by polymeric nanoparticles programming T cells in situ. (Parayath,2020)
IVT mRNA CAR-T Cells Targeting Solid Malignancies
Summary: The study evaluated the safety and efficacy of adoptive cell therapy with IVT mRNA mesothelin-specific CAR-T cells in 6 patients with chemotherapy-refractory metastatic PDAC. After infusion, transient CAR expression was identified in patients' blood, leading to the proliferation of new immunoglobulin G proteins. The findings support the anticancer effect of mesothelin-specific IVT mRNA CAR-T Cells.
Summary: The study through the purification of mRNA encoding CAR to generate robust CAR-T cells. And in vivo investigations employing a leukemia mouse model found that T cells electroporated with pure mRNAs, regardless of nucleoside alteration, achieved the most effective 100-fold decrease of leukemic load.
If you want to learn more details about our in vivo IVT mRNA-based CAR-T engineering service, please don’t hesitate to contact us.
Frequently Asked Questions
Q1. What should be paid attention to in the production process of IVT mRNAs for in vivo CAR-T engineering development?
A: Many aspects need to pay attention to in the production process, UTR types, polyA length, and codon optimization are very important to improving mRNA stability and translation efficiency. Based on our years of experience, we recommend the right solution according to the needs of the client's project for in vivo IVT mRNA-based CAR-T engineering project.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION