CAR-T cells are created by re-engineering an individual's T lymphocytes in the lab to express receptors that can recognize tumor antigens. Once these cells have been proliferated ex vivo, they are re-infused into the individual to identify and attack tumor cells.
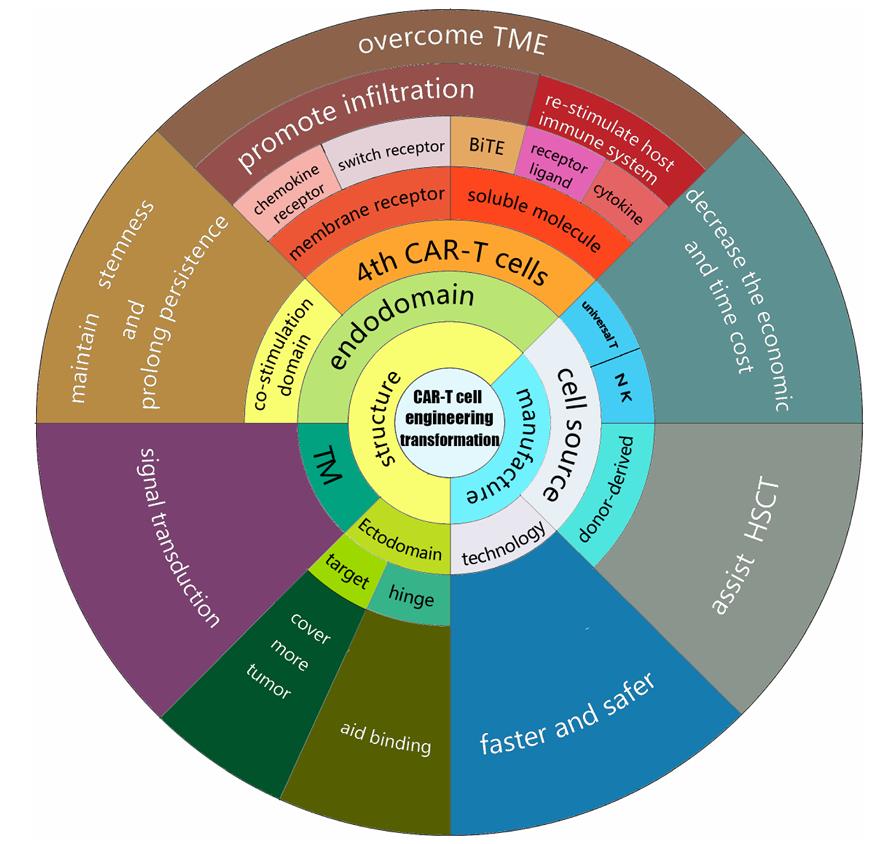 Fig. 1 The various aspects of CAR-T cell design and development.1
Fig. 1 The various aspects of CAR-T cell design and development.1
When the CAR binds to an antigen on the surface of tumor cells, it activates the T cells, prompting them to proliferate and release cytokines, thereby enhancing the immune response. Activated CAR-T cells can directly kill tumor cells primarily by releasing cytotoxic molecules such as perforin and granzymes. The design of CAR-T cells aims to enhance their persistence and memory within the body, allowing for a rapid response in the event of tumor recurrence. Gene editing technologies are being utilized to modify CAR-T cells, enhancing their anti-tumor capabilities and their ability to withstand the tumor microenvironment. Next-generation CAR-T cells (such as fourth-generation CAR-T cells) are designed to secrete immune-modulating factors that improve their survival in the tumor microenvironment and counteract the immunosuppressive effects of tumors.
Creative Biolabs provides a comprehensive suite of services, beginning with the development of processes and test methods that are tailored to meet specific project needs while adhering to GMP standards. We offer flexible production scales and process designs for the manufacturing of CAR-T cells. We also ensure that our methods are validated by focusing on specificity, accuracy, precision, and other essential parameters. Additionally, we conduct stability studies that encompass long-term, accelerated, and shipping stability assessments.

By employing closed and automated cell culture equipment, Creative Biolabs aligns with global industry standards, ensuring efficiency and consistency. Our cell manufacturing facility is designed to achieve higher cell proliferation rates and effectively address challenges related to low positive rates. With extensive experience in manufacturing clinical samples and handling IND submissions, Creative Biolabs welcomes collaboration and invites you to reach out for partnership opportunities.
We specialize in providing IND-grade manufacturing support for innovative anti-cancer cell therapies, spanning from initial material collection to the final infused cell product. Whether you are advancing an autologous or allogeneic therapy, we act as your trusted manufacturing partner — delivering flexible, scalable, and quality-driven services to help you progress into the clinical phase.
"Partnering with Creative Biolabs enabled us to move from starting material to first patient dose in record time. The project management team kept us fully informed, and the hand-off to clinical supply went smoothly." — Senior Director, Immuno-Oncology Biotech.
"Their flexible manufacturing platform allowed us to scale seamlessly from Phase I to Phase II without changing the contract manufacturer. That transition alone saved us months and significant cost." — Head of Cell Therapy Development.
"The technical expertise and quality-control support were outstanding. We felt confident submitting our IND knowing the manufacturing partner had deep experience and full analytical capabilities." — Chief Scientific Officer, Gene-Engineered Cell Therapy Company.
What are the components of quality control in the context of CAR cell manufacturing?
Quality control in CAR cell manufacturing includes routine tests such as appearance, pH, osmolality, cell counts, and cell viability. It also involves safety tests, which assess CAR gene copy number, endotoxin levels, sterility, and mycoplasma presence, as well as impurity assessments.
How does your team ensure the efficient progression of a client's project?
Our team ensures the efficient progression of a client's project through expert project management, which involves handling intricate details and ensuring the timely and accurate completion of all stages. This approach reduces the burden on the client's internal team, allowing them to focus on other critical tasks.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION