The clinical translation of NK cell-based immunotherapies is often hindered by limitations in achieving robust, large-scale expansion while maintaining high product purity, potent cytotoxicity, and in vivo persistence. Creative Biolabs' Irradiated Feeder Cell-Induced NK Cell Expansion Technology overcomes these barriers by utilizing irradiated, engineered feeder cells to deliver synergistic activation signals through receptors. This approach enables greater than thousand-fold NK cell expansion under optimized conditions, significantly enhancing cytolytic activity, cytokine secretion, and functional persistence, all while eliminating the tumorigenic risks associated with conventional feeder systems.
Feeder cells, through cell surface receptor-ligand interactions and cytokine support, provide a potent ex vivo stimulus that drives the robust activation and proliferation of NK cells. This approach not only enables the attainment of clinically relevant NK cell numbers from limited starting materials but also profoundly enhances the cells' cytotoxic potential and facilitates efficient genetic engineering. The irradiated feeder cell-induced NK cell expansion technology, wherein feeder cells are γ-irradiated prior to co-culture to prevent their proliferation and potential contamination of the final product, serves as the cornerstone for the clinical-scale manufacturing of both unmodified and genome-edited NK cell therapies.
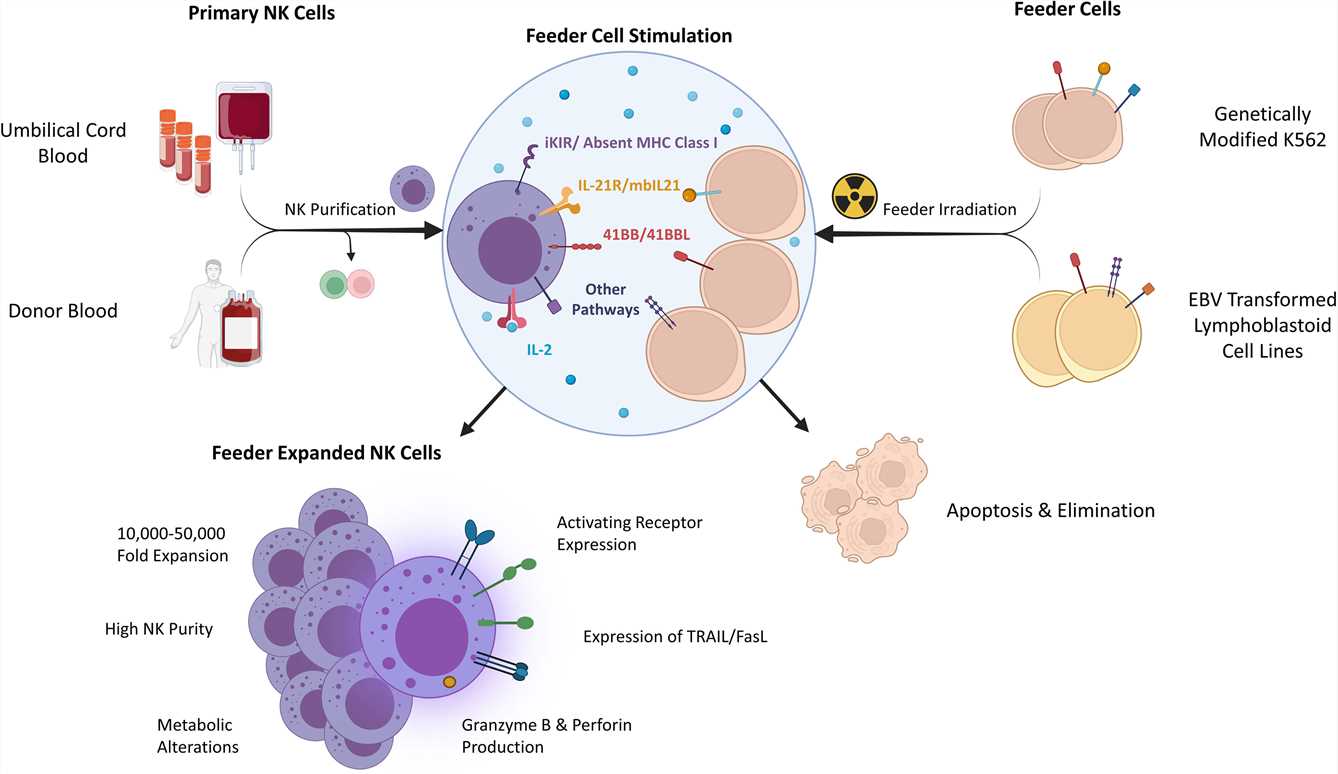 Fig.1 Key factors and traits of feeder-expanded natural killer cells.1
Fig.1 Key factors and traits of feeder-expanded natural killer cells.1
Creative Biolabs' Irradiated Feeder Cell-Induced NK Cell Expansion Technology provides a platform for the large-scale production of highly functional NK cells. It employs irradiated feeder cells to provide a potent, synergistic activation signal while eliminating the safety concerns of conventional methods. We achieve robust, thousand-fold expansions of NK cells with superior cytotoxic potency, cytokine production, and enhanced persistence.
Our service provides a robust, scalable solution to overcome the critical bottlenecks in clinical NK cell therapy. By combining proven biological principles with advanced engineering and optimized protocols, we deliver high-purity, high-potency, and high-yield NK cell products ready to address both hematological and solid tumor challenges.
Our detailed workflow is designed to maximize NK cell yield and purity while maintaining full traceability and process control.
Required starting materials:
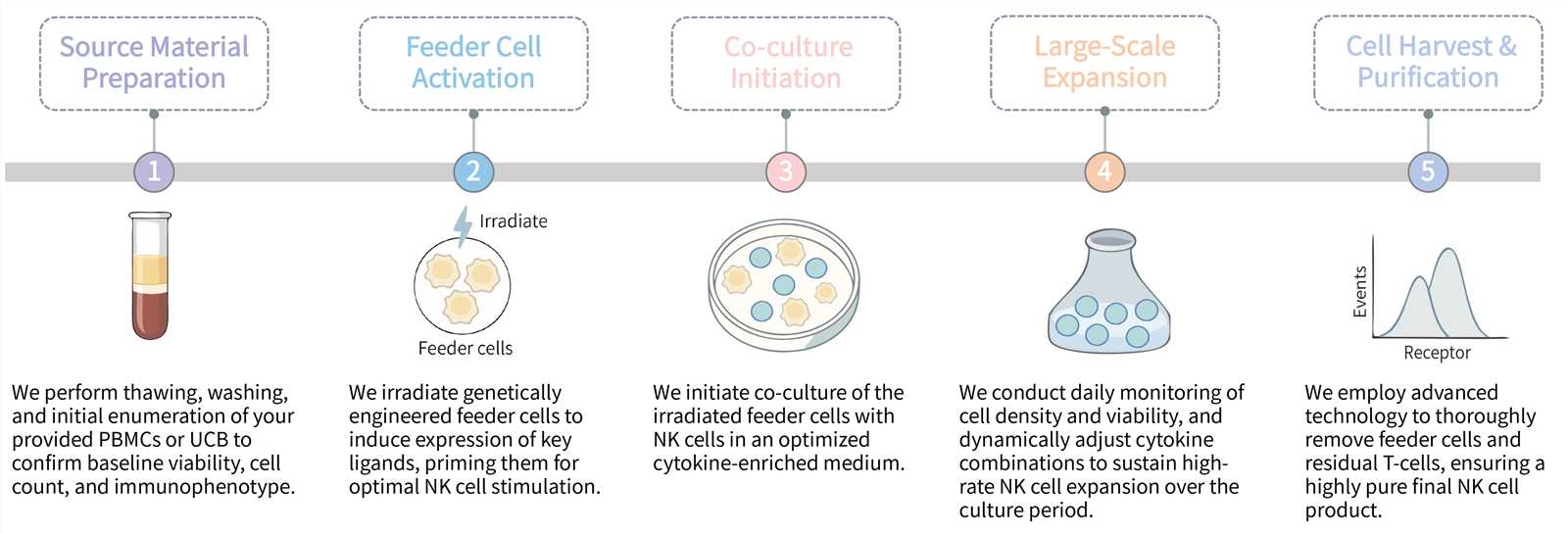
Final Deliverables:
Is your feeder cell system suitable for generating both allogeneic and autologous NK products?
Yes, our platform is highly adaptable. We offer established protocols for expanding NK cells derived from both allogeneic sources (e.g., UCB, donor PBMCs) and autologous patient-derived PBMCs. This flexibility allows you to seamlessly transition your therapeutic strategy.
How does your expansion protocol compare to simple cytokine-driven methods in terms of purity?
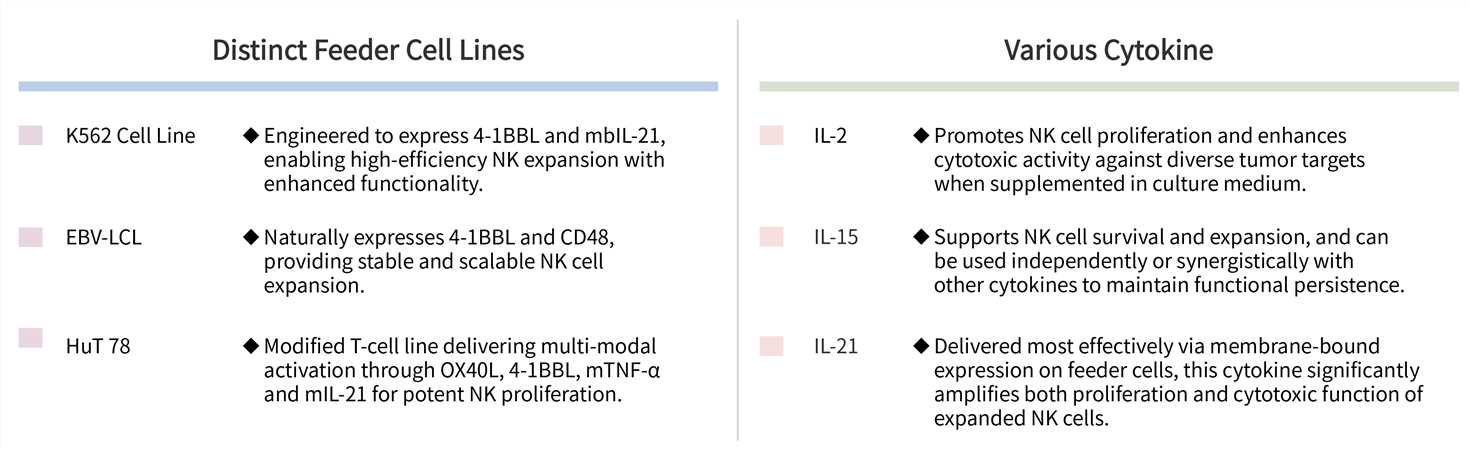
Cytokine-only methods often result in significant T cell contamination, which can compromise product safety. Our irradiated feeder cells are engineered to specifically express potent NK-activating ligands, which synergistically promote NK cell proliferation while actively suppressing T lymphocyte expansion, guaranteeing a much higher final purity.
Creative Biolabs' NK cell expansion platform is built on two decades of immunological expertise, directly addressing the limitations identified in foundational research, such as the functional decline associated with NK cell irradiation. We achieve functional superiority and maximized persistence by optimizing co-stimulatory inputs and employing highly characterized feeder systems.
"Using Creative Biolabs' Irradiated Feeder Cell-Induced NK Cell Expansion Technology in our research has significantly improved the transduction efficiency of our novel CAR construct. The highly proliferative state of the expanded cells made lentiviral integration dramatically more successful compared to standard IL-2 culture methods, which was a critical bottleneck for us."— Dr. H***g, Cell Therapy Developer.
"The cells expanded using Creative Biolabs' system showed superior cytotoxic function against solid tumor cell lines in vitro, necessary for our planned combination therapy study. We compared them against an irradiated NK cell equivalent and found the Creative Biolabs product retained significantly higher serial killing capacity, addressing the core limitation of older cell lines."— A. T***e, Lead Research Scientist.
We invite you to explore how our advanced NK cell manufacturing platform can accelerate the development of your therapeutic pipeline. To unlock its potential for your specific programs, we welcome the opportunity to engage in a technical consultation.
Partner with our team to unlock the full potential of your project.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION