We construct chemically programmed switch CARs offering external drug control. Our services include design, engineering, and validation, addressing safety concerns, reducing uncontrolled activity, and providing precise therapeutic regulation.
KIR-based CAR design is a pioneering approach in the field of CAR-T therapy. It involves the incorporation of Killer-cell Immunoglobulin-like Receptors (KIRs) into the CAR construct. KIRs are a family of cell surface proteins found on natural killer (NK) cells and a subset of T cells. These receptors play a pivotal role in regulating the immune response by interacting with Human Leukocyte Antigens (HLAs) on the surface of target cells.
By integrating KIRs into CAR-T cells, our KIR-based CAR design service at Creative Biolabs aims to enhance the specificity, efficacy, and safety of CAR-T therapy. This innovative approach provides a means to fine-tune the immune response and overcome certain limitations of conventional CAR-T therapies, especially in the context of solid tumors and the challenges posed by the tumor microenvironment.
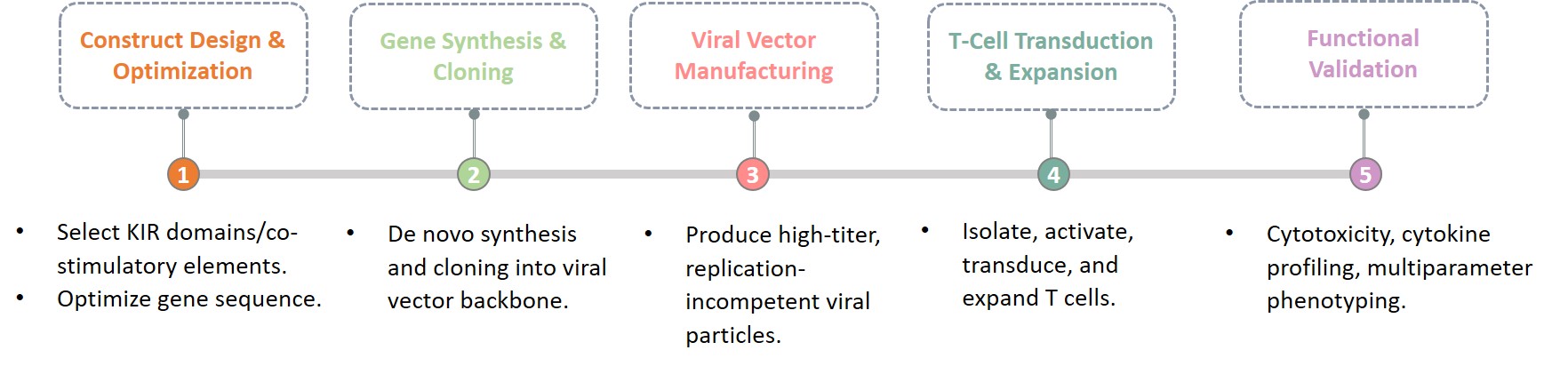
Our expert team of scientists and engineers is proficient in designing, optimizing, and validating KIR-based CAR-T constructs. The key components of our KIR CAR construction services include:
A CAR constructed using this natural multi-chain immunoreceptor design offers several compelling reasons for showing sustained efficacy in CAR-T resistant tumor models, ultimately leading to complete tumor remission:
Please contact us to advance the success of your research.
Creative Biolabs utilizes a state-of-the-art, non-infringing technology stack covering the entire KIR-CAR development process, from initial gene design through final functional validation, ensuring a high-quality, translational product.
| Vector Construction and Synthesis Platforms | Cellular and Functional Validation Platforms |
|---|---|
|
|
This rigorous, validated platform workflow ensures that your KIR-CAR T-cell candidates are built for maximum potency and regulatory compliance.
Connect with us today to obtain detailed details and tailor-make your service package.
What makes KIR-based CARs different from conventional CAR designs?
KIR CARs leverage KIR signaling domains, which can offer stronger persistence, balanced activation, and reduced exhaustion compared to traditional CAR formats. This makes them especially attractive for enhancing NK- or T-cell–based therapies.
Can I use KIR CARs for both T cells and NK cells?
Yes. While KIR domains are naturally associated with NK cell biology, they can also be integrated into CAR-T designs to provide alternative signaling pathways. We help you adapt the KIR CAR architecture to the effector cell type that best fits your project goals.
How do KIR CARs compare to second and third-generation CARs?
Traditional CAR generations typically rely on CD28 or 4-1BB co-stimulatory domains. KIR CARs represent an alternative signaling pathway that may complement or outperform these depending on the therapeutic context. We help you evaluate whether a KIR-based design is more suitable for your target disease compared to conventional CAR formats.
We construct chemically programmed switch CARs offering external drug control. Our services include design, engineering, and validation, addressing safety concerns, reducing uncontrolled activity, and providing precise therapeutic regulation.
Our team designs CD3ε-based CARs utilizing natural TCR signaling pathways. Services include vector engineering and validation, addressing weak CAR activation and enhancing signal strength for improved antitumor responses.
Creative Biolabs engineers adnectin-based CARs as antibody alternatives. Our services include scaffold design, optimization, and testing, addressing limitations of scFvs and providing stable, versatile targeting options for innovative cellular therapies.
Creative Biolabs is your trusted partner for engineering the next generation of adoptive cell therapies. Our KIR CAR construction service provides the critical engineering, quality control, and functional validation required to transform complex scientific concepts into high-impact clinical candidates. Ready to advance your immuno-oncology program with superior cell engineering? Inquire now to receive detailed specifications and customize your service package.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION