Lipopolysaccharide (LPS) stimulates immune responses by interacting with the membrane receptor CD14 to induce the generation of various cytokines. LPS challenge evaluates the animal's ability to respond to an inflammatory stimulus by mounting an acute phase response. As one of the well-recognized animal model research partners, Creative Biolabs offers LPS-induced cytokine release models for preclinical research of drug candidates.
LPS, the most abundant component within the cell wall of gram-negative bacteria, is one of the main etiological factors in the pathogenesis of many diseases. LPS consists of the lipid A moiety and a polysaccharide component which is made up of the O-specific chain, an outer core and an inner core. LPS can stimulate the release of interleukin 8 (IL-8), IL-6, tumor necrosis factor (TNF)‑α and other inflammatory cytokines in various cell types, leading to an acute inflammatory response towards pathogens. Bacterial LPS has been widely used in models studying inflammation as it mimics many inflammatory effects of cytokines. The cellular receptor transducing the LPS signal has been identified as toll-like receptor 4 (TLR4). Binding of LPS to TLR4 leads to the activation of NF-κB through the recruitment and activation of IL-1R kinase (IRAK), MyD88, TNFR associated factor 6 (TRAF-6), and NADPH oxidase (Nox).
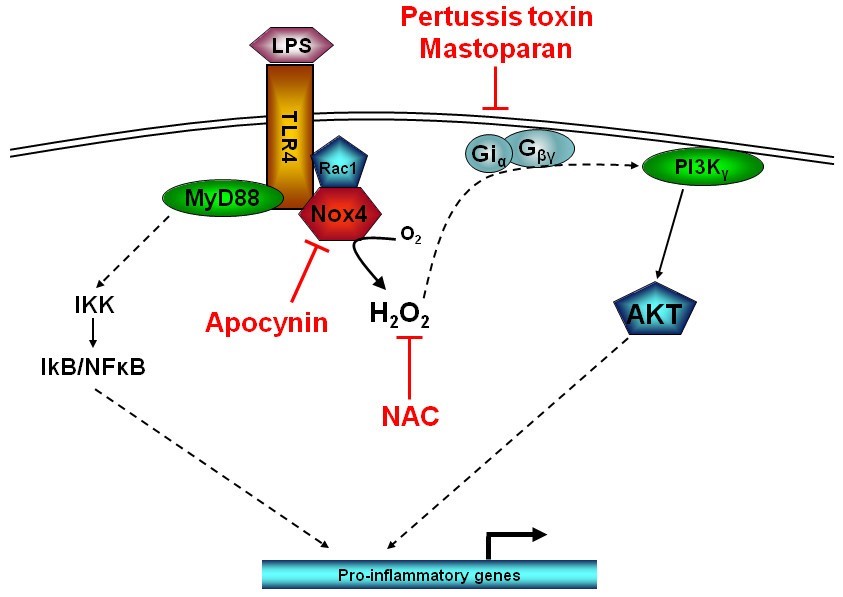 Fig.1 Schematic representation of LPS-induced PI3K activity.1
Fig.1 Schematic representation of LPS-induced PI3K activity.1
The LPS animal model has several essential advantages such as technical ease and high reproducibility, particularly in the inflammatory response elicited. Shortly after LPS administration, high levels of pro-inflammatory cytokines are released and can be measured in circulating serum. In assessing the impact on acute inflammation, the LPS model is the most suitable, as systemic effects are easily identifiable and measurable.
Each animal such as rat or mouse receives the LPS injection. At specified time points post-LPS challenge, blood will be collected for cytokine analysis. Creative Biolabs has the capability to deliver test items by multiple routes, such as oral to assess systemic action or inhaled to understand systemic contribution to the efficacy of lung-delivered compound. Creative Biolabs also offers the customized LPS-induced disease model such as LPS-induced pulmonary neutrophilia model, or LPS-Induced rodent sepsis model to investigate the effect of targeting mechanisms.
Experts at Creative Biolabs are available to help provide guidance throughout your project. Please contact us to offer more information about your requirements and we will contact you to discuss how we can support your journey to market.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION