At Creative Biolabs, we pride ourselves on providing comprehensive One-Stop Allogeneic Cell Therapy Development Services. Among our specialized offerings is the development of marrow-infiltrating lymphocytes (MILs), harnessing their unique bone marrow residency and potent antitumor properties.
MILs are a subset of T cells that naturally reside within the bone marrow. They have gained significant interest in the field of cancer immunotherapy due to their unique properties and potential therapeutic applications.
Key Characteristics of MILs:
Bone Marrow Residency: MILs are found in the bone marrow, where they play crucial roles in immune surveillance and maintaining immune homeostasis.
Tumor Infiltration: MILs have a natural ability to infiltrate the bone marrow, making them particularly relevant for targeting hematologic malignancies such as multiple myeloma and certain leukemias.
Enhanced Immune Function: MILs often exhibit potent anti-tumor activity and enhanced immune functions compared to peripheral blood lymphocytes (PBLs). This includes higher cytotoxicity against tumor cells and greater cytokine production.
Creative Biolabs is proud to offer comprehensive MIL development services, designed to support the advancement of innovative cell therapies targeting various cancers.
The combination of MILs and CAR-T therapy is a promising area of research with the potential to overcome some of the limitations of current CAR-T therapies. Our key directions:
Our services encompass every stage of MIL-based therapy development, ensuring high-quality, efficient, and regulatory-compliant processes.
| 1 | Cell Source Identification and Procurement | Donor Screening and Selection: Comprehensive donor health screening, genetic matching, and procurement of high-quality bone marrow samples. |
| 2 | MILs Isolation and Characterization |
Cell Isolation: Advanced techniques for isolating MILs from bone marrow, ensuring high purity and viability. Phenotypic Characterization: Flow cytometry and other assays to characterize the surface markers and phenotypic properties of MILs. Functional Assays: In vitro assays to assess the cytotoxic activity, cytokine production, and proliferation capacity of isolated MILs. |
| 3 | Genetic Engineering |
CAR Construct Design and Development: Custom design of CARs tailored to target specific cancer antigens. Transduction and Expansion: Lentiviral or retroviral transduction methods to introduce CAR constructs into MILs, followed by expansion protocols to obtain sufficient cell numbers for therapeutic use. |
| 4 | Testing |
In Vitro Efficacy Testing: Co-culture assays to evaluate the cytotoxicity of CAR-MILs against target cancer cell lines. Animal Models: In vivo testing in relevant animal models to assess the efficacy, persistence, and safety of CAR-MILs. Biodistribution Studies: Tracking and analyzing the distribution of CAR-MILs within animal models to understand their migration and targeting capabilities. |
| 5 | Manufacturing and Scale-Up |
Manufacturing: Production of CAR-MILs to ensure quality and safety. Process Development: Optimization of manufacturing processes to improve yield, reduce costs, and ensure consistency. Cryopreservation and Storage: Development of protocols for the cryopreservation and long-term storage of CAR-MILs without compromising their functionality. |
This study illustrates the potent antitumor effects of activated MILs. The data demonstrate that MILs when activated, exhibit superior cytotoxic activity against cancer cells compared to PBLs. This enhanced efficacy is attributed to the unique microenvironment of the bone marrow, which primes MILs to recognize and target tumor cells more effectively. Additionally, activated MILs produce higher levels of key cytokines, further boosting their antitumor response. These findings underscore the therapeutic potential of MILs in targeting bone marrow-residing cancers and highlight their role as a promising avenue for advanced cancer immunotherapy.
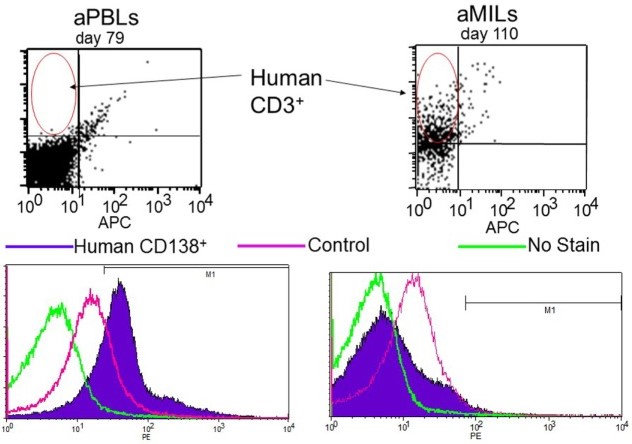 Fig.1 Activated MILs exert a potent antitumor MILs.1
Fig.1 Activated MILs exert a potent antitumor MILs.1
1. How are MILs isolated from bone marrow?
We use advanced isolation techniques to extract MILs from bone marrow samples. This process ensures high purity and viability of the isolated cells, making them suitable for therapeutic applications.
2. What is the advantage of using MILs over PBLs in cancer therapy?
MILs are naturally primed to recognize and target tumor cells within the bone marrow, leading to enhanced antitumor activity. They also exhibit greater persistence and efficacy in the bone marrow microenvironment compared to PBLs.
3. How are CAR constructs introduced into MILs?
We utilize lentiviral transduction or other gene-editing methods to introduce chimeric antigen receptor (CAR) constructs into MILs. This process involves optimizing transduction efficiency and expanding the modified cells to therapeutic levels.
4. What preclinical testing services do you offer for MIL-based therapies?
Our preclinical testing services include in vitro efficacy assays, in vivo testing in animal models, and biodistribution studies to evaluate the effectiveness, safety, and targeting capabilities of CAR-MILs.
5. How do you ensure the long-term viability of MILs during storage?
We develop and utilize cryopreservation protocols that maintain the functionality and viability of MILs during long-term storage, allowing for their use in future therapeutic applications.
Our expertise in isolating, engineering, and optimizing MILs for targeted cancer therapy ensures that we will meet your specific research and therapeutic needs. Whether you are advancing novel treatments for hematologic malignancies or exploring innovative immunotherapies, Creative Biolabs is your trusted partner. Contact us today to discover how our MIL Development Service can accelerate your path to effective and personalized cancer therapies.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION