Serving as a powerful in vitro tool, the MHC-associated Peptide Proteomics (MAPPs) assay provides significant value for immunogenicity risk assessment of biotherapeutics through the direct identification of naturally presented T cell epitopes. Creative Biolabs' MHC-associated Peptide Proteomics (MAPPS) Assay Service is supported by a robust, automated platform that guarantees data reproducibility and systematicity. We deliver a thorough evaluation of potential T cell epitopes in candidate molecules, pinpoint immunogenic hotspots, and provide key evidence to guide early drug development, thereby furnishing a crucial foundation for de-risking candidates.
MAPPs is a mass spectrometry-driven immune-peptidomics platform that directly captures and deciphers the repertoire of peptides naturally processed and presented on HLA class II molecules by professional antigen-presenting cells. The workflow pulses candidate biotherapeutics onto human dendritic cells, immunoaffinity-enriches the resulting HLA-peptide complexes, and subjects them to high-resolution LC-MS/MS, thereby closing an experimental loop from living cell to actionable peptide sequence.
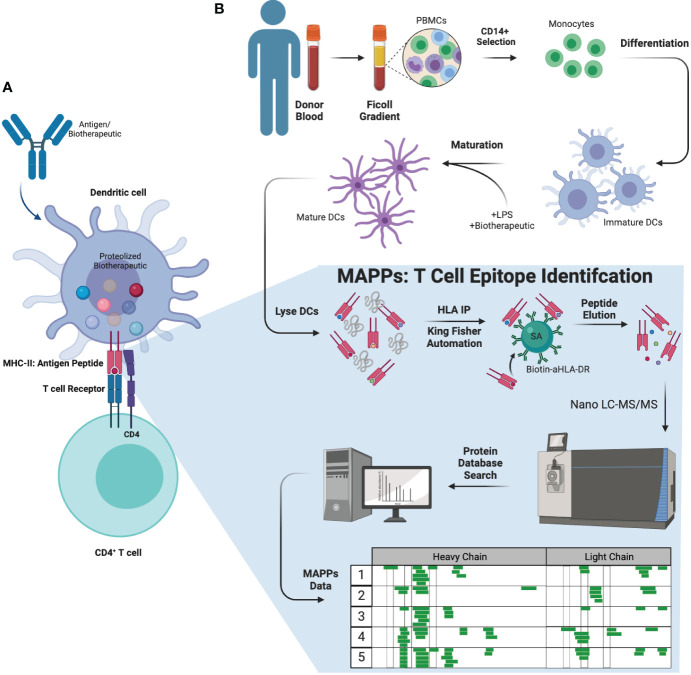 Fig.1 MAPPs assay: a workflow for predicting ADA responses to biologics.1
Fig.1 MAPPs assay: a workflow for predicting ADA responses to biologics.1
Creative Biolabs' MHC-associated Peptide Proteomics (MAPPS) Assay Service delivers a critical profile of a biologic's immunogenic potential through direct profiling of MHC Class II-associated peptides. Our dataset allows for the accurate mapping of T cell epitopes, informing strategic decisions early in development to minimize immunogenicity risks and guide the rational design of optimized biotherapeutics.
Our MAPPS assay is a cornerstone service within our immunogenicity assessment portfolio, delivering the data-driven precision essential for modern drug development. We provide tailored solutions to address the unique challenges of your project, whether you are investigating novel antibodies, vaccine candidates, or complex therapeutic proteins.
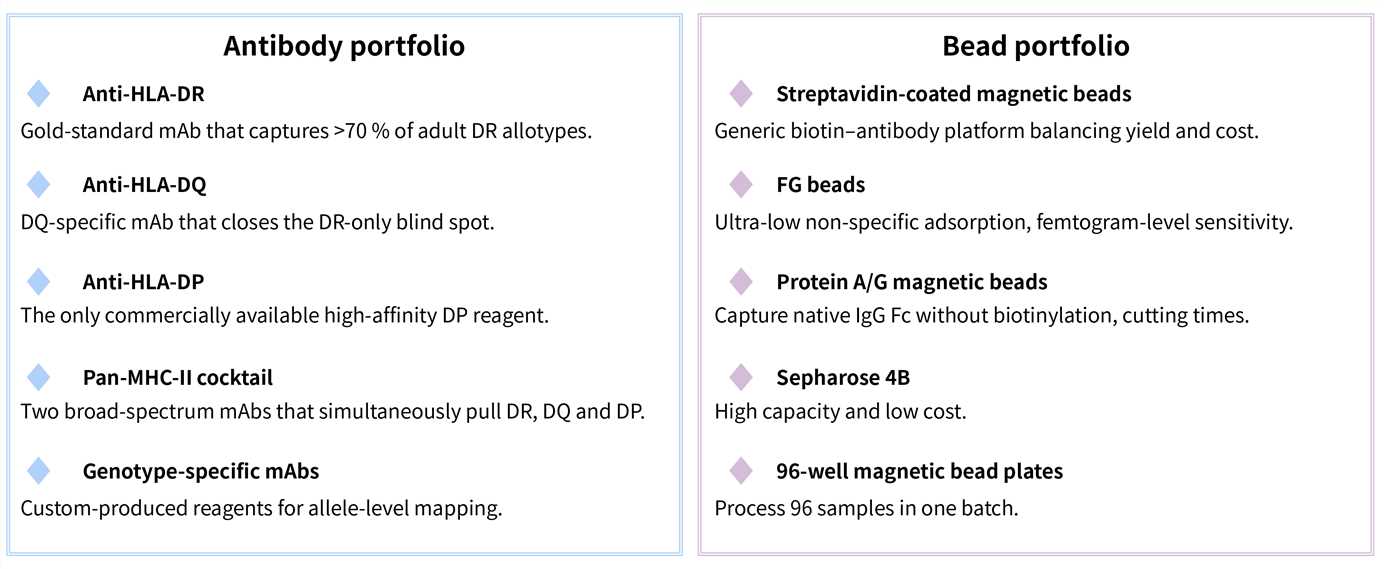
Required Starting Materials:
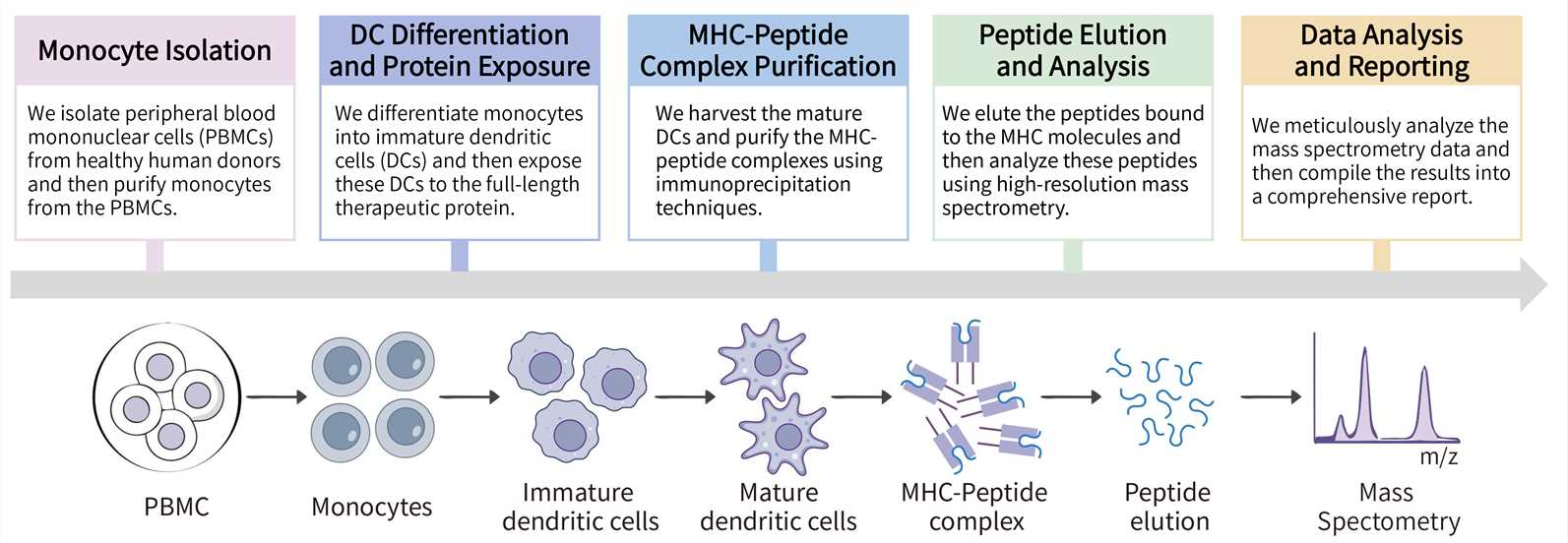
Key Steps Involved:
Final Deliverables: We deliver a final package that provides you with the full mass spectrometry dataset, including a curated list of identified peptides and their sequences, ready for downstream analysis.
How does MAPPS differ from conventional in-silico epitope prediction?
Algorithm-based approaches estimate MHC-binding affinity and frequently over-score immunogenic potential. MAPPS delivers empirical, cell-derived peptide-HLA landscapes, furnishing a biologically validated and quantitatively precise read-out of true antigen presentation.
What is the minimal protein input for MAPPS?
The actual quantity is project- and protein-dependent (size, expression level, post-translational modifications). Please consult our specialists and we will generate a customized sample-requirement table after a brief feasibility review.
Our MAPPS assay offers a distinct advantage over traditional in silico prediction methods by delivering experimentally validated evidence of which peptides are actually presented by human cells. Our approach significantly reduces the risk of overpredicting immunogenicity and yields a more accurate immunogenicity profile.
"By employing Creative Biolabs' MAPPS Assay Service, we gained critical insights into naturally processed and presented peptides, which substantially de-risked our lead candidate selection. The dataset not only validated our predictions but also uncovered unexpected epitopes, greatly enriching our antigen understanding." J**n S.
"The comprehensive MHC-associated peptide profiling provided by the MAPPS assay enabled us to move forward into clinical development with greater confidence. The direct correlation between protein sequence and actual MHC presentation proved decisive, streamlining our candidate selection process and conserving significant time and resources." M**a K.
"We validated Creative Biolabs' MAPPS results against internal T-cell proliferation assays and observed strong concordance, confirming its reliability as a predictive immunogenicity tool. This service offers a distinct advantage over conventional methods for early-stage immunogenicity profiling." L**y W.
At Creative Biolabs, we are dedicated to supporting your research with cutting-edge, reliable services designed to accelerate your project's success. Our specialists are available to provide a detailed consultation on how our MAPPS assay service can be customized to address your unique requirements.
Reach out to our specialists today to explore potential applications for your project.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION