The Mixed Lymphocyte Tumor Reactivity (MLTR) assay is a sophisticated in vitro co-culture system designed to model and quantify tumor antigen-specific T cell immune responses. Leveraging our robust, high-throughput screening platform, Creative Biolabs' Mixed Lymphocyte Tumor Reactivity Assay Service provides a standardized and reproducible solution for evaluating cell-based immunotherapies. Our optimized platform ensures the generation of highly reliable, multiparameter data on T cell activation, proliferation, and cytotoxic function with minimal variability. We thereby empower you with critical preclinical insights to de-risk decision-making, optimize candidate selection, and significantly accelerate immuno-oncology drug development pipeline.
Mixed Lymphocyte Reactivity (MLR) is the classic in vitro paradigm in which immune-competent cells from two allogeneic donors are co-cultured, triggering proliferation and cytokine release through reciprocal recognition of foreign MHC molecules. MLTR extends this platform by incorporating autologous or allogeneic tumor cells, generating a tripartite "immune-tumor" co-culture that faithfully recapitulates tumor-antigen–specific T cell priming and effector phases. The model offers an early, integrated read-out of therapy-induced expansion, phenotypic polarization, and cytotoxic capacity of tumor-reactive T cells, supplying both mechanistic insight and dose-ranging data for subsequent in vivo studies. Consequently, implementing a standardized, high-sensitivity MLTR assay is an indispensable checkpoint in the cancer immunotherapy drug-discovery pipeline.
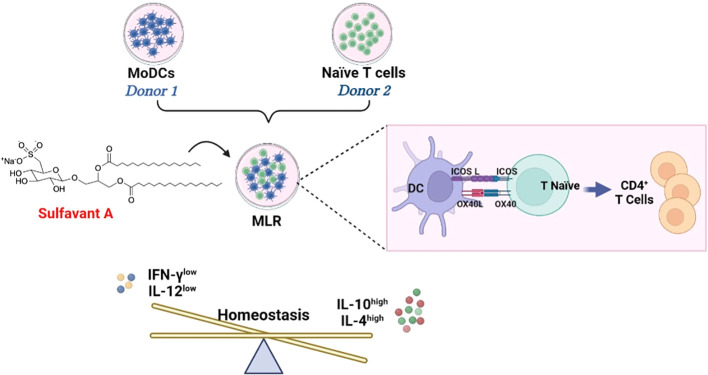 Fig.1 Sulfavant A induces a tolerogenic shift in mixed lymphocyte reactivity.1
Fig.1 Sulfavant A induces a tolerogenic shift in mixed lymphocyte reactivity.1
Creative Biolabs' Mixed Lymphocyte Tumor Reactivity Service is designed to provide clear and actionable insights into the immune interactions between lymphocytes and tumor cells. Utilizing a robust experimental platform, our service systematically evaluates T cell activation, proliferation, and cytotoxic responses upon exposure to tumor antigens, key aspects in the development of novel cancer immunotherapies. Through precise and reliable detection methods, the assay effectively assesses the potential of your therapeutic candidates to induce anti-tumor immune responses.
Our service leverages state-of-the-art techniques to provide an in-depth assessment of T-cell cytotoxic efficacy, delivering actionable insights for your research.

Required Starting Materials:
Key Steps Involved:
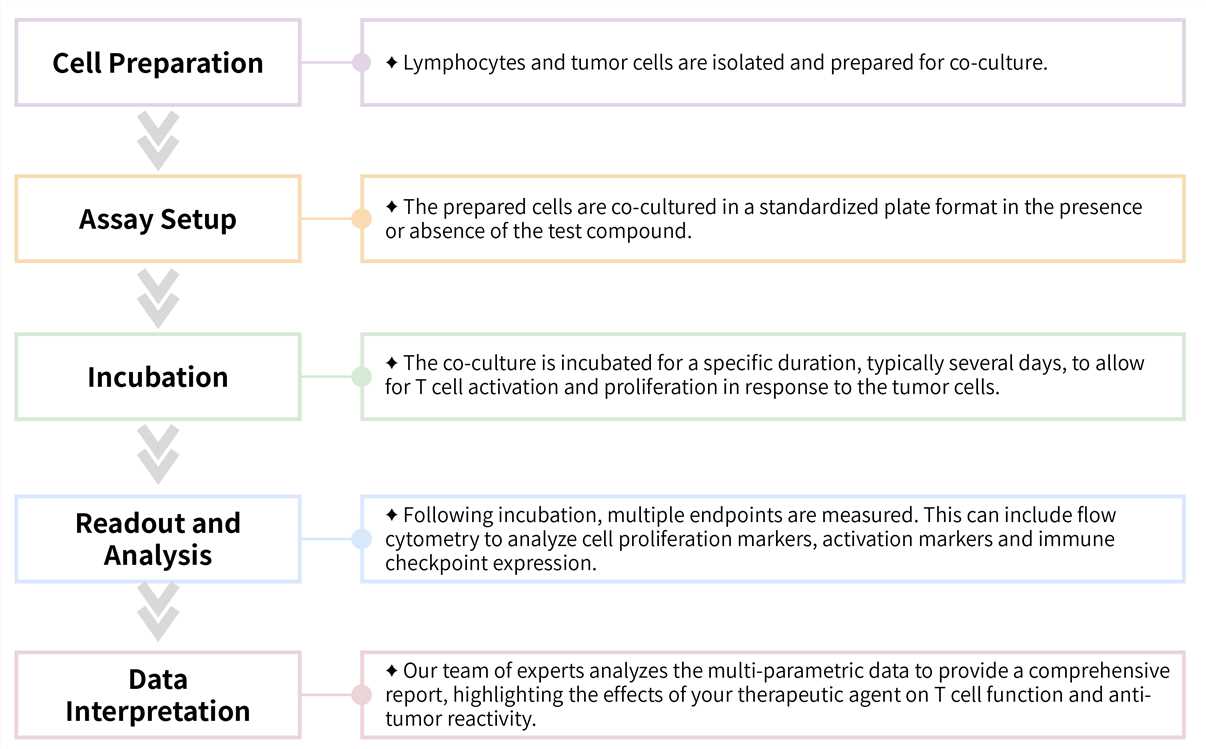
Final Deliverables: Upon completion, you will receive a detailed report including raw data, analyzed graphs (e.g., dose-response curves), and a professional interpretation of the results.
What cell types are compatible with your MLTR assay?
The service is compatible with various cell inputs, such as Peripheral Blood Mononuclear Cells (PBMCs), isolated T cell populations, and primary or established tumor cell lines. Our scientific team can provide guidance on selecting the optimal combination to align with your specific research objectives.
How is experimental variability controlled in primary cell-based assays?
We employ rigorously standardized protocols and a high-throughput platform to ensure data robustness. This system enables efficient testing across multiple replicates and compound concentrations, generating statistically powerful and reproducible results.
Our MLTR assay delivers validated, high-quality data to advance your immuno-oncology research. Our robust and high-throughput platform accelerates the screening of therapeutic candidates by providing reliable and reproducible results with significant gains in efficiency and cost-effectiveness.
"Integrating Creative Biolabs' MLTR Assay Service into our research workflow greatly enhanced the characterization of our lead candidate's mechanism of action. The multi-parametric output, including proliferation and cytokine release metrics from a single assay plate, provided critical insights that supported deeper mechanistic understanding." Dr. B***n, R&D Director.
"Creative Biolabs' MLTR assay delivered highly specific and reproducible data, which played a key role in verifying the anti-tumor efficacy of our engineered T cell therapy. The assay's robustness made it an essential component of our validation pipeline." A. J***s, Senior Scientist.
"In a comparative assessment with in-house methods, Creative Biolabs' high-throughput MLTR platform demonstrated superior efficiency. The capability to screen numerous compounds and concentrations concurrently resulted in significant savings in time and resources." Dr. S***t, Project Lead.
Unlock key insights into your immuno-oncology candidates with our Mixed Lymphocyte Tumor Reactivity Assay Service. Contact our experts to discuss how our customized solutions can advance your project.
Unlock Tailored Solutions with Our Expertise – Request Your Quote Now.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION