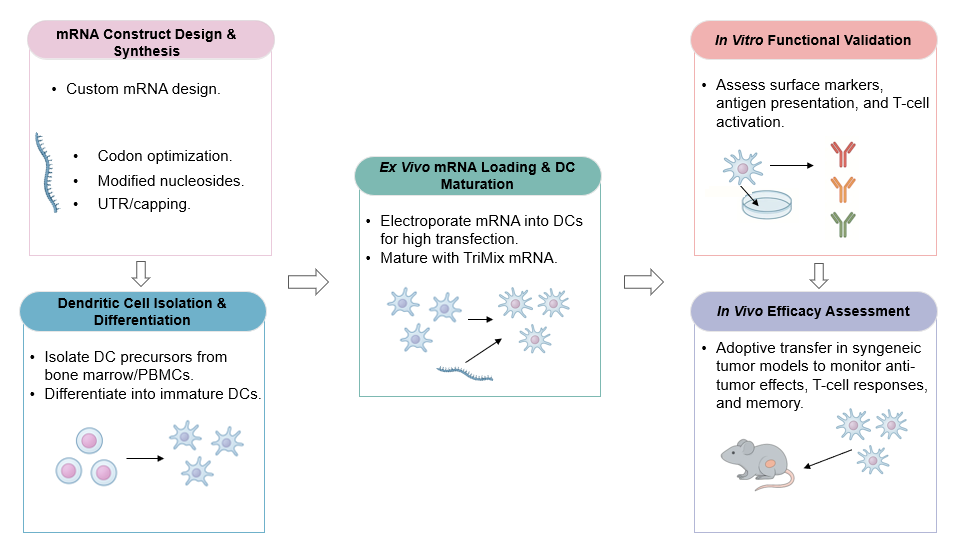
Creative Biolabs provides an mRNA-loaded dendritic cell (DC) development service that utilizes advanced ex vivo mRNA engineering techniques and multi-epitope antigen presentation platforms to accelerate therapeutic development, enhance targeted anti-tumor responses, and support the generation of effective cancer immunotherapies against heterogeneous tumors.
Dendritic cells (DCs) are master regulators of the immune system, uniquely capable of initiating and amplifying anti-tumor T cell responses. Modern immunotherapy capitalizes on its role to overcome tumor-induced immune suppression. The advent of mRNA technology offers unprecedented precision in programming DCs with multiple tumor antigens and immunomodulatory signals. This approach bypasses limitations of direct in vivo delivery and leverages DCs' inherent ability to present antigens and activate T cells effectively.
Creative Biolabs delivers a comprehensive solution for advancing cancer immunotherapy. Our service provides precisely engineered dendritic cells, optimized through ex vivo mRNA loading, to tackle tumor heterogeneity and immune evasion. We enable the generation of potent, multi-faceted anti-tumor immune responses, including robust CD8+ cytotoxic T lymphocyte (CTL) activity and broad epitope recognition. Clients can expect custom-designed mRNA constructs encoding multiple tumor antigens and co-stimulatory molecules, ensuring tailored and highly effective cellular vaccines. Our rigorous functional validation guarantees the therapeutic relevance of the engineered DCs.
Advanced design with codon optimization, nucleoside mods, and capping for stability/efficiency; supports multi-epitope tumor/neoantigen constructs.
High-efficiency mRNA loading, including co-stimulatory molecules for DC maturation.
MHC I/II-targeted mRNA constructs to stimulate CD8+/CD4+ T cells for anti-tumor responses.
In vitro/in vivo assays for DC maturation, T-cell activation, and anti-tumor efficacy in animal models.
mRNA stability/encapsulation strategies (e.g., LNP principles) to ensure pre-transfection integrity.
Summary: This study designed mRNA lipid nanoparticle vaccines targeting highly conserved HIV protease cleavage sites to enhance cellular immunity, with a cold-chain compatible formula. Mouse trials showed it triggered specific CD8 memory T cells systemically and at mucosal sites, with little CD4 T cell activation, confirming its potential as an effective HIV preventive vaccine.
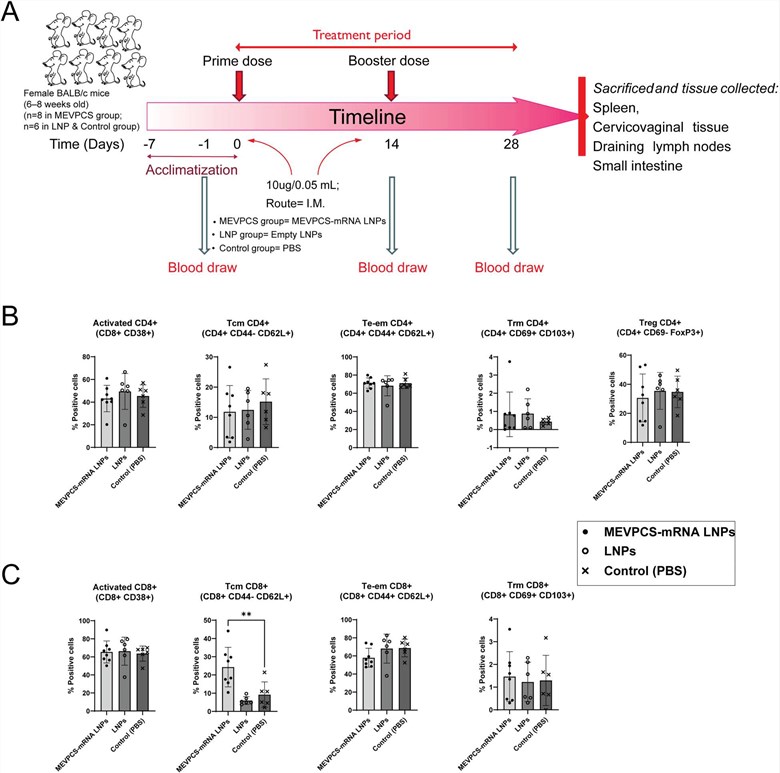 Fig.1 Investigations into the in vivo efficacy of mRNA vaccines.1
Fig.1 Investigations into the in vivo efficacy of mRNA vaccines.1
Q1: How does this service compare to direct in vivo mRNA vaccine delivery?
A1: While direct in vivo mRNA delivery has shown great promise, our ex vivo DC engineering approach offers distinct advantages. It provides precise control over the antigen payload and DC activation status, potentially reducing systemic toxicities and overcoming immune suppression often found in the tumor microenvironment.
Q2: What kind of support can I expect after the deliverables are provided?
A2: Creative Biolabs is committed to your success. Our support extends beyond final delivery, offering scientific consultation to assist with data interpretation, integration into your downstream studies, and troubleshooting. We aim to be a long-term partner in your immunotherapy development journey.
Q3: Can your engineered DCs be used in combination with other immunotherapies?
A3: Absolutely. Our mRNA-loaded DCs are designed to prime robust T-cell responses, making them ideal candidates for combination therapies, such as with immune checkpoint inhibitors. By enhancing the initial immune recognition, they can synergize with other modalities to achieve superior therapeutic outcomes. We encourage discussion on potential combination strategies.
At Creative Biolabs, our mRNA-loaded DC development service represents a fusion of cutting-edge mRNA technology and advanced cellular engineering. We empower researchers and pharmaceutical companies to overcome the complexities of cancer immunology, delivering precisely tailored, multi-epitope engineered dendritic cells designed to elicit robust and lasting anti-tumor immunity.
"The ability to precisely deliver multiple epitopes via engineered DCs has facilitated our studies on overcoming tumor immune escape. Creative Biolabs' expertise in designing the mRNA constructs and their efficient electroporation technique is unparalleled, saving us considerable development time." – Pr. Sh Ka.
"Creative Biolabs provided a highly reproducible and efficient service for generating our customized mRNA-DCs. Their attention to detail in quality control and the clarity of their deliverables allowed us to confidently integrate these cells into our therapeutic vaccine strategy. The consistent performance exceeded our expectations." – Ms. Aa Lu.
Feel free to reach out at any time to obtain detailed insights into our mRNA-loaded DC development service. Our expertise spans custom mRNA design, ex vivo DC engineering, and functional validation. Let's discuss how our tailored solutions can align with your project goals and advance your research in tumor immunotherapy.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION